Flower wasp coupling

Flower wasps are most distinctive and obvious when they're mating.
Attached end to end, the winged male carries the ant-like female in flight and supports her as she feeds.
(Thynnidae: Thynninae)
From August through to April, such sightings are common here in the forest. Many species, a range of sizes, adopting a variety of postures.
I decided it was time I learnt more about the impressive structures involved in their coupling. Which bits belong to which individual? What role do the different parts play in the mating process? And might this knowledge help me identify our local species, at least to something beyond 'subfamily Thynninae'?
This blog is rather lengthy, presented in four parts:
mating behaviour – field observations of coupling initiation.
male terminalia – dissection of one specimen to illustrate the component parts, including a summary table.
the twist – visualising the inversion of the phallus, with the aid of a schematic.
the action, up close – revisiting a dozen species in the field, in detail.
Part 1: mating behaviour
Single males are a common sight. The larger species noisily patrol forest paths, flying a couple of metres above ground. Smaller males weave back and forth amongst the low vegetation, perching often to preen and rest. And, of course, they’re attracted to open, nectar-filled flowers such as Leptospermum and Eucalyptus.
In stark contrast, females spend most of their time below ground (Ridsdill Smith, 1970). They seek hosts such as beetle grubs on which to lay their eggs, although for most species nothing is known of their particular life histories. Whatever their subterranean targets, they are well adapted for digging through soil. Strong legs, ridged abdomen, protective mandibles, no wings, no ocelli, small eyes – all perfect for a fossorial life, but not so helpful for insects that depend on an occasional sugary drink! For this they need assistance from a male.
Occasionally we spot a lone female, having emerged from the soil and clinging to low vegetation as she awaits the arrival of a winged mate.
More often, though, I locate the site of her imminent emergence by the circling of rival males. If I’m lucky, and patient, I witness ‘the catch’ – the moment a male arrives and grabs hold of the female. Details vary. Here are a few examples.
Example 1: the ‘grab-and-fly-off’ technique
A female calls from her perch on low vegetation. A male arrives and, after a quick inspection, wraps his legs around her body and carries her off in flight. They will perch somewhere nearby and couple.
Example 2: the ‘couple-first, fly-later’ technique
A female calls from her perch on low vegetation. A large male arrives and, after a quick inspection, they couple and remain at the same perch … at least for a while. Later they fly off, still in copula.
Example 3: the ‘grounded’ technique
A male lands on the the ground and pokes about amongst the leaf litter. A female makes several fleeting appearances as they investigate one another. Eventually he hauls her out into the open where they couple. After several minutes of mating and feeding, she disappears underground and he flies off alone.
Part 2: the male terminalia
The coupling mechanism must be strong and secure, yet flexible. Mating in flower wasps is highly active. It is common to see the female twisting about freely, suspended in mid air. Pairs may remain in copula for prolonged periods (Alcock, 1981), including in flight, and some females are relatively large.
Why knowledge of the bits matters
Reproductive structures are commonly included in morphological descriptions and classifications of wasps and other insects. Keys to genera of thynnines are no exception, commonly referencing ‘parameres’, ‘hypopygium’ and ‘epipygium’.
Interest in the coupling apparatus of thynnine wasps extends beyond pure taxonomy. A recent evolutionary study describes the role selective pressures have played on diversification of the subfamily (Semple et. al., 2021).
The bottom line for me – it's well worth getting to know what’s what!
Excerpt from Snodgrass, R.E. 1941 (‘The male genitalia of Hymenoptera’), p. 12.
A little labwork required
As luck would have it, my reference collection includes a large male flower wasp (#2304A). Although he is long dead, the parts that matter are well preserved.
Such is the nature of most insects. The muscles, nerves and internal organs disappear, but the exoskeleton (cuticle) resists decay. Indeed most taxonomic studies of insects rely upon this fact. Dried insects housed in museums are critically important to taxonomic revisions, including the original (type) specimens which in some species are hundreds of years old. By comparison, #2304A is almost fresh!
Discovered, dead, on 5th April, 2023. Collected and stored dry for future reference (specimen label 2304A).
So I set about dismantling the terminalia of #2304A. Carefully. Piece by piece. And with reference to the general structure of hymenopteran genitalia (Snodgrass, 1941).
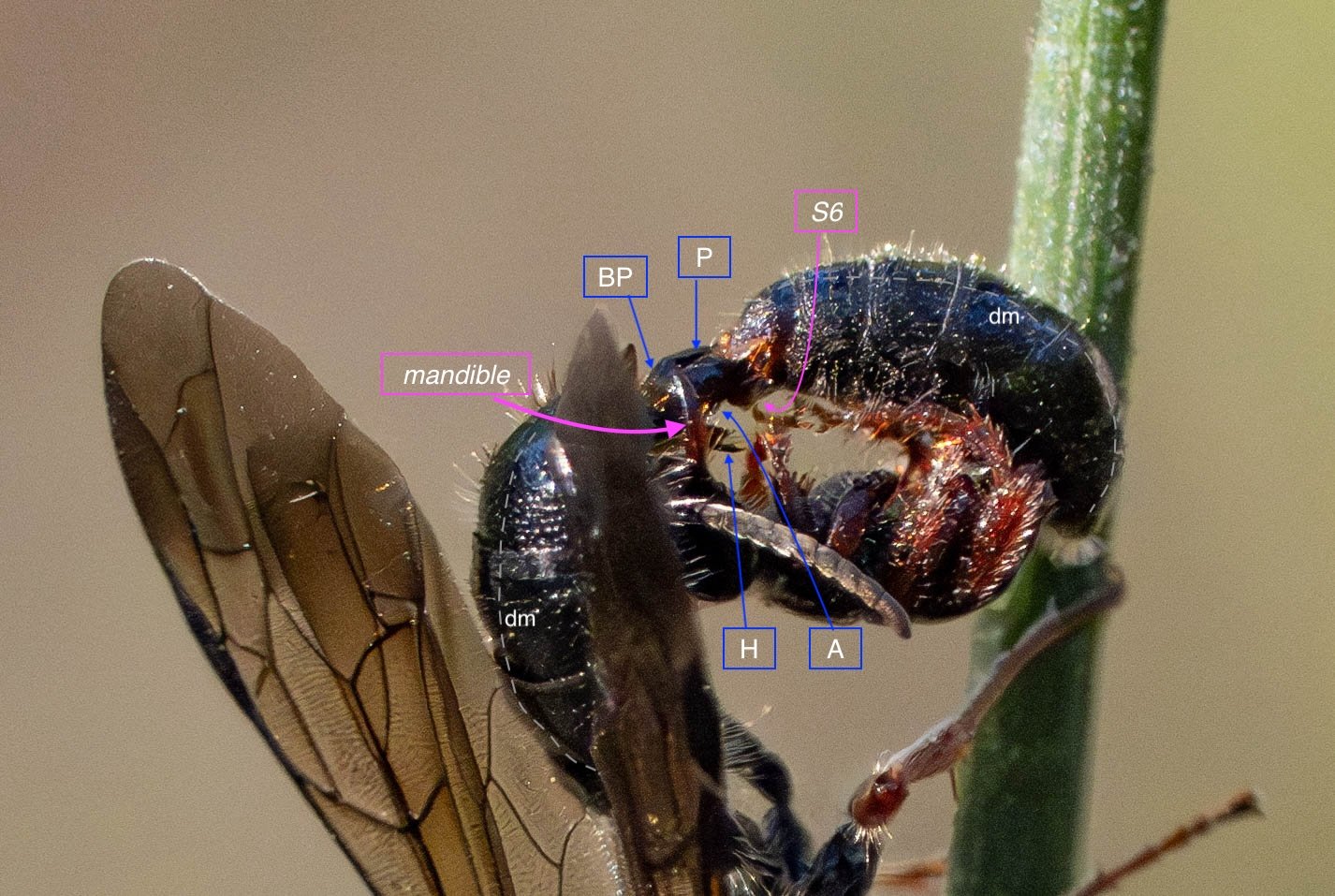
An overview
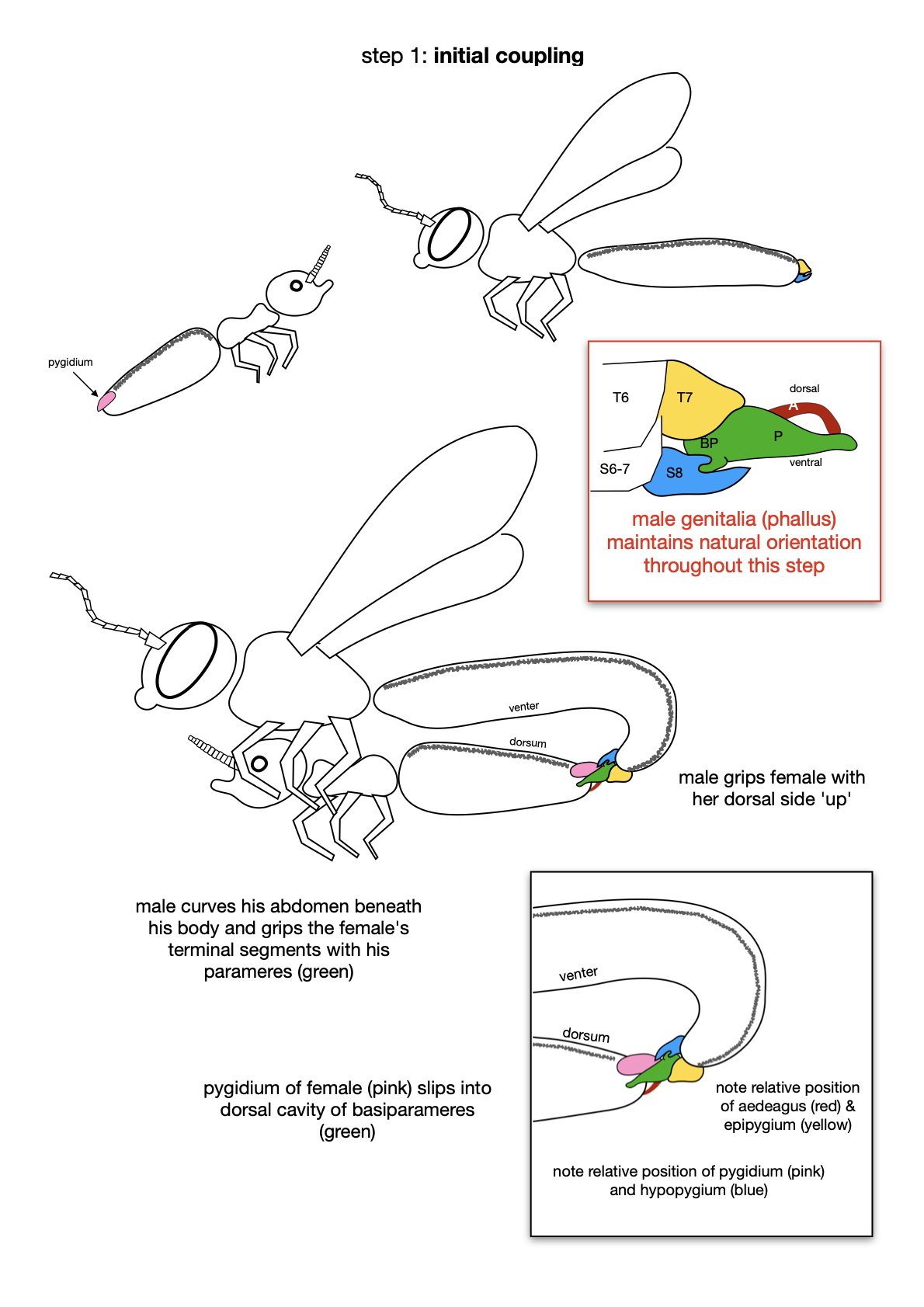
Before discussing the genitalia in detail, it is important to consider their location and housing.
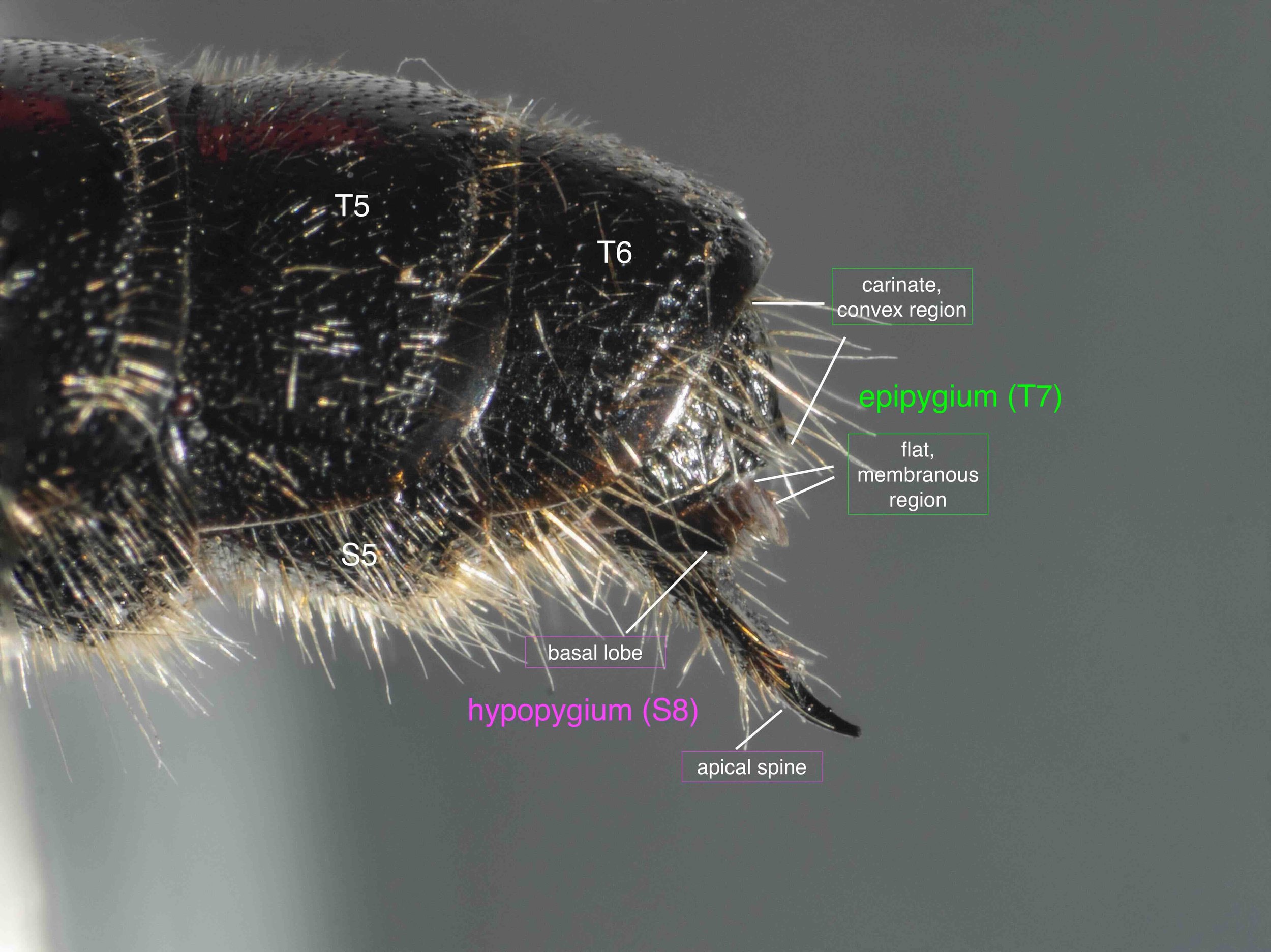
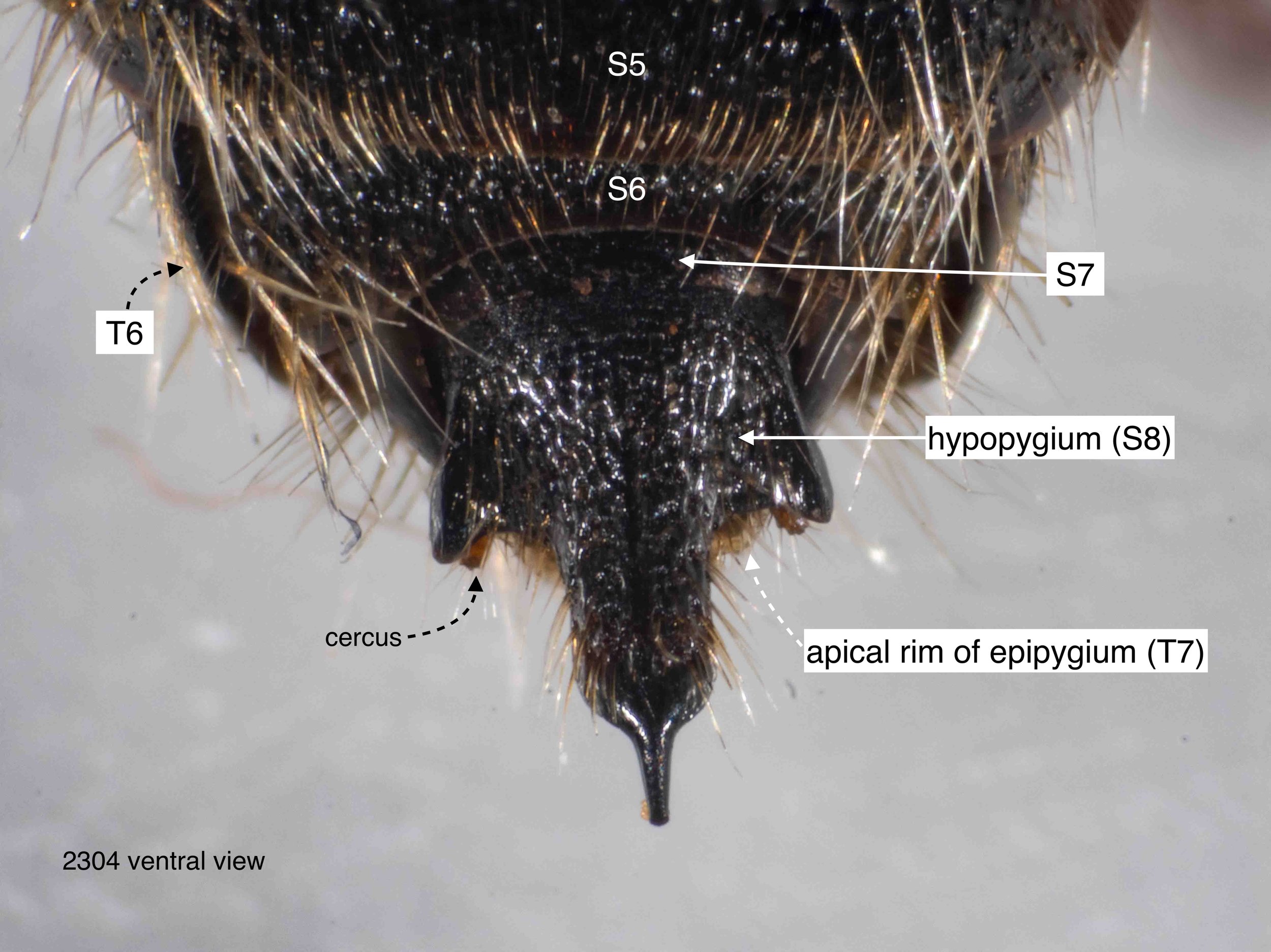
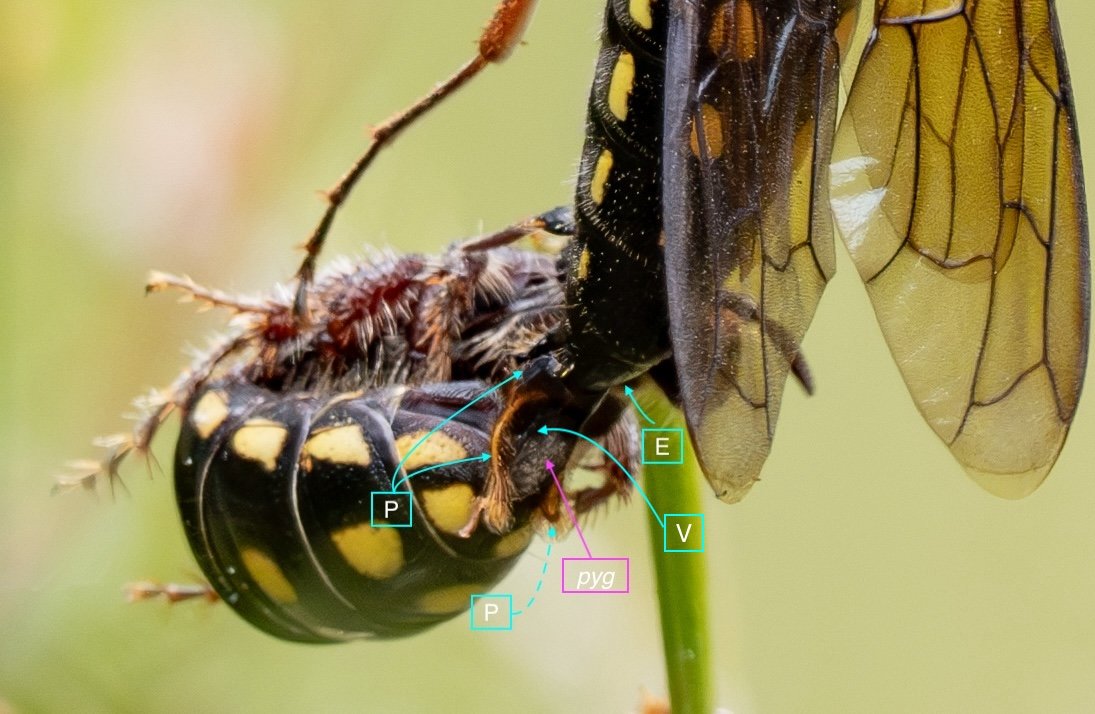
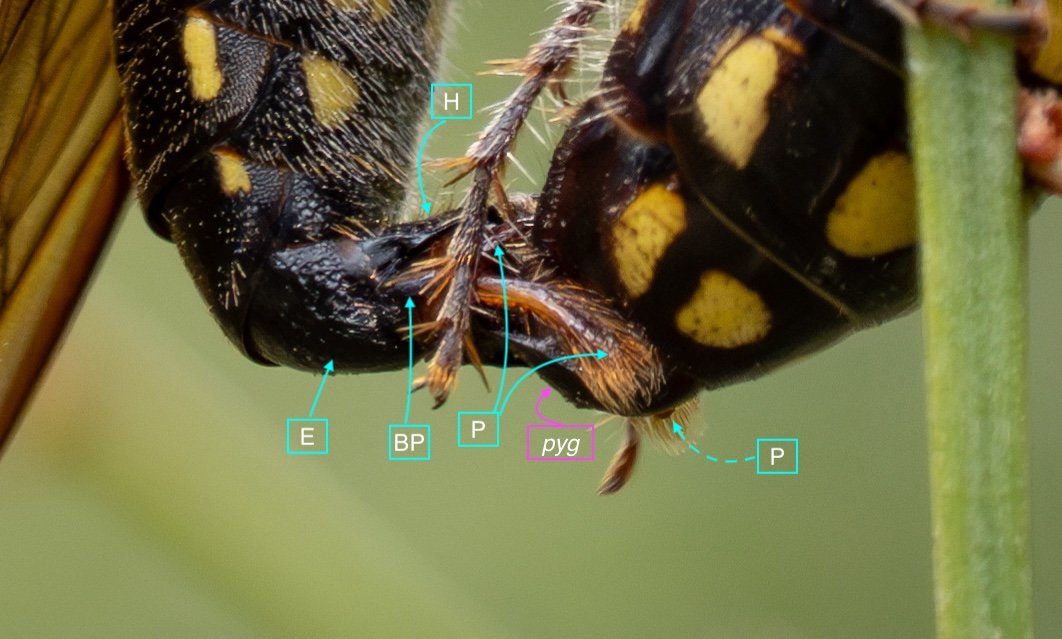
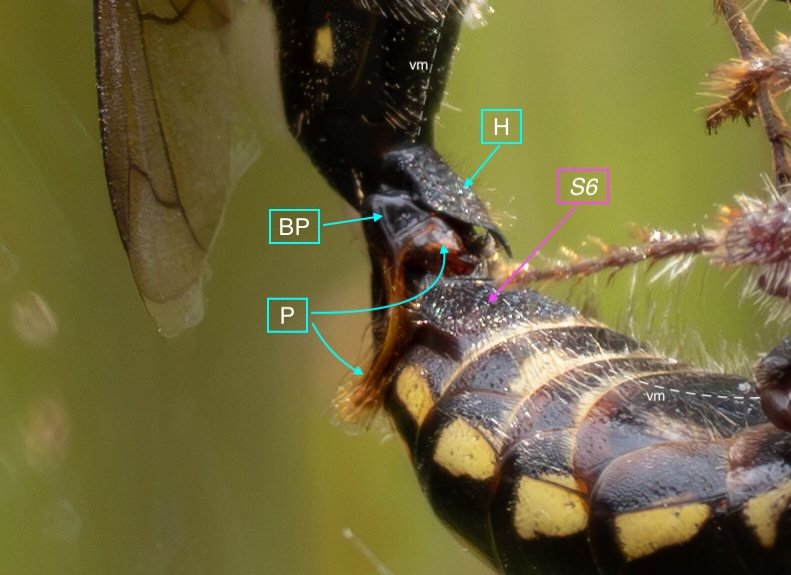
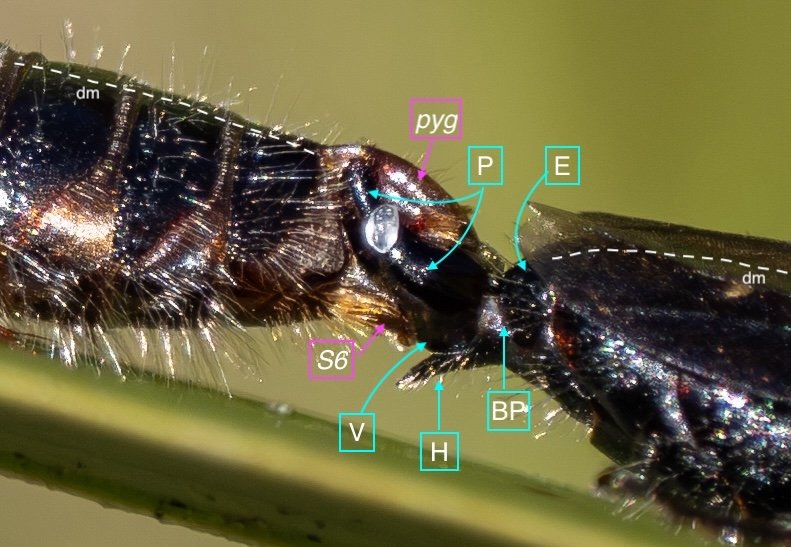
The last visible sclerites of the body, the epipygium and hypopygium, enclose the genital cavity.
Within the genital cavity sits the genitalia, collectively called the phallus.
During copulation, the phallus is extended and the covering sclerites are pushed apart.
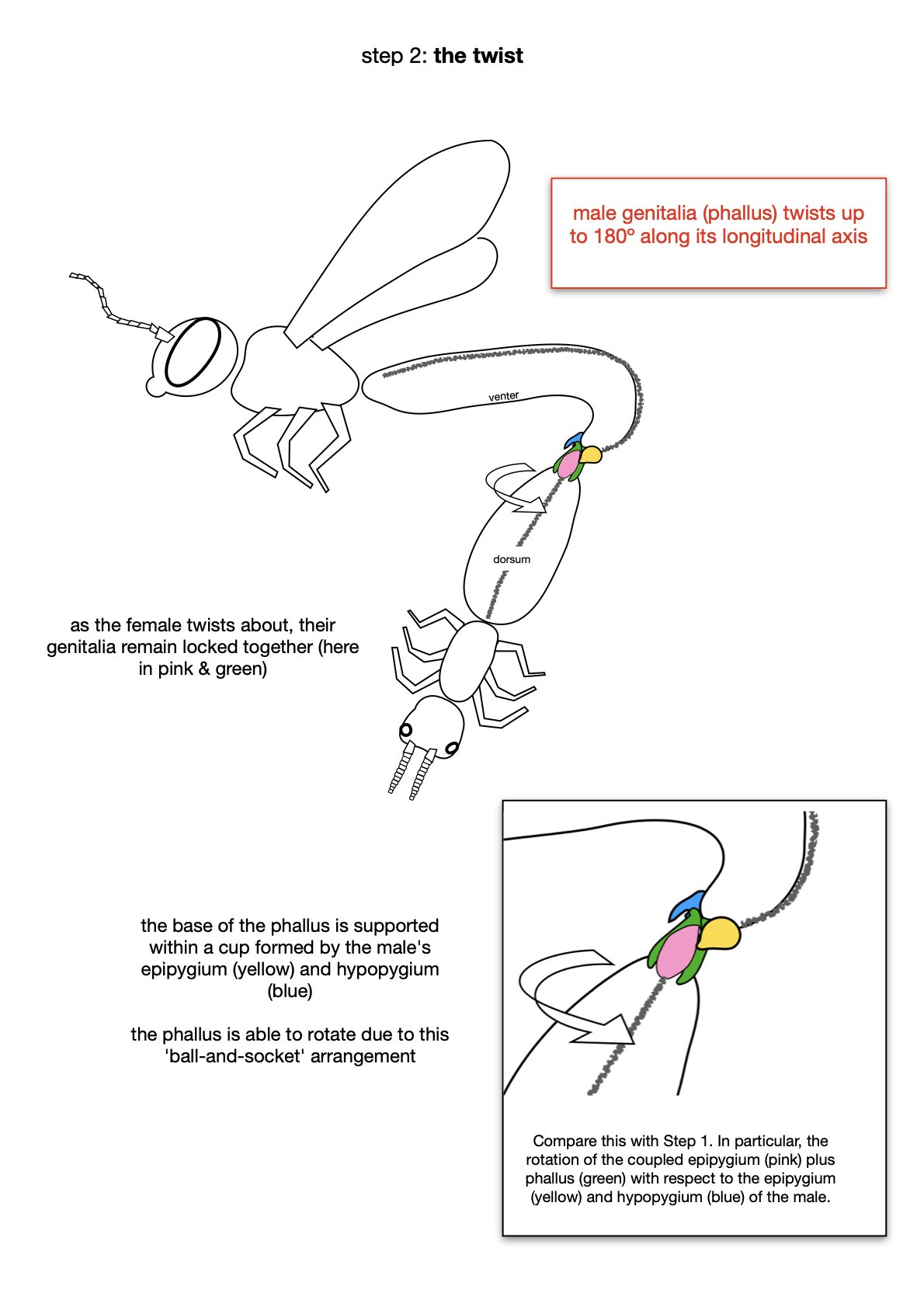
The entire phallus can be raised (dorsally) or lowered (ventrally). It also rotates, such that ventral becomes dorsal and vice versa. I’ll come back to details of this inversion (‘the twist’) in Part 3.
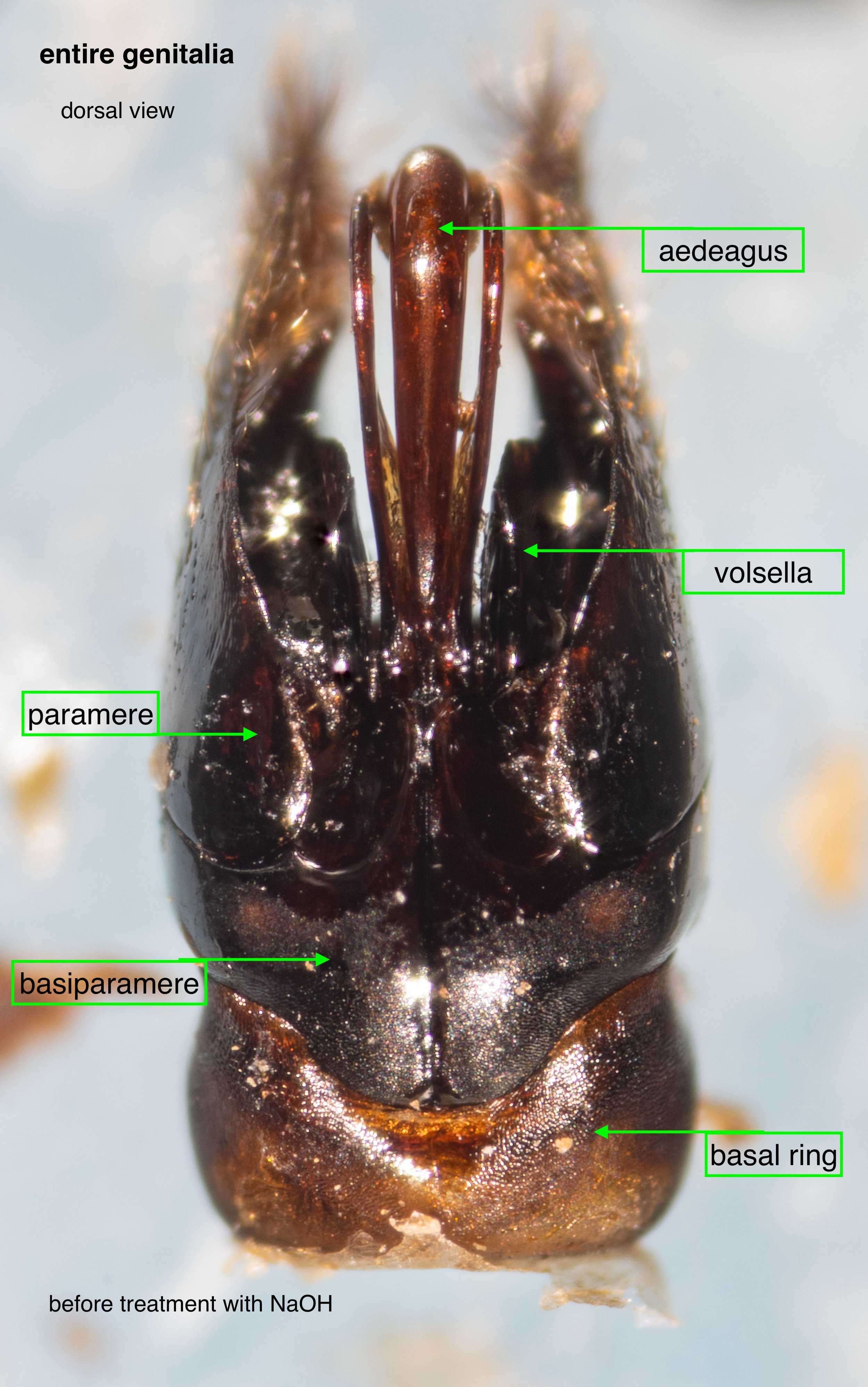
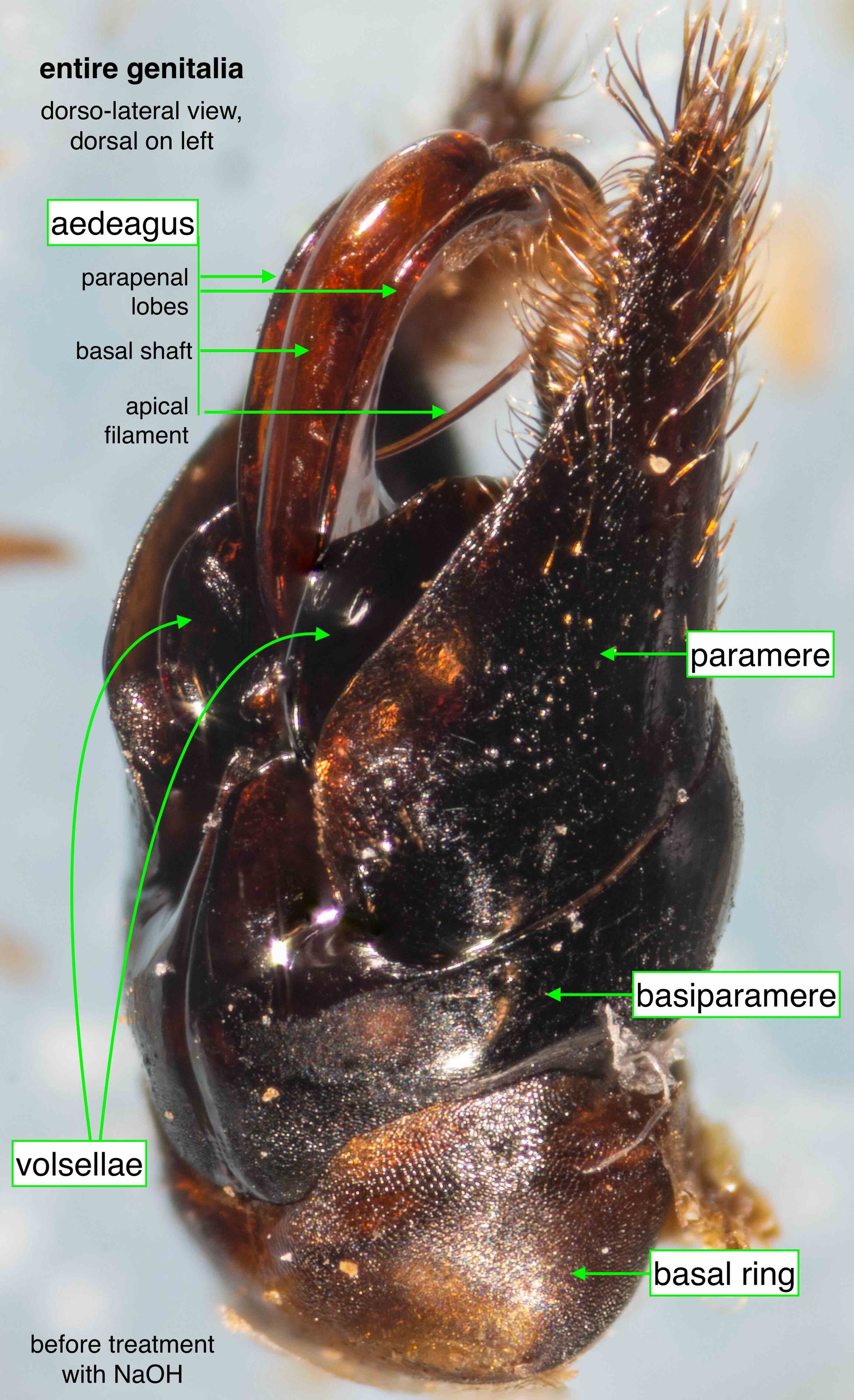
In the following seven images I unpack the phallus from the surrounding sclerites.
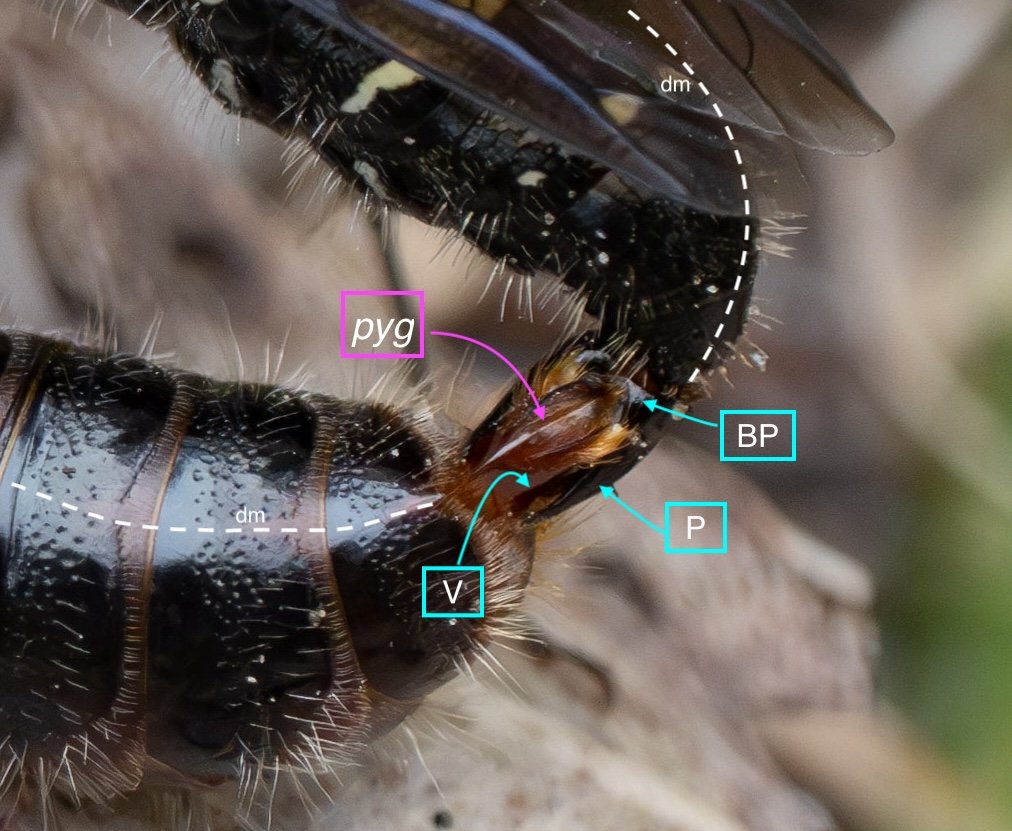
The epigyium & hypopygium
After cleaning away muscles and other dried tissues, the shape and surface architecture of the epipygium and hypopygium become apparent. These details differ between genera, and so will certainly be helpful when I come back to species identification … but that’s for another day.
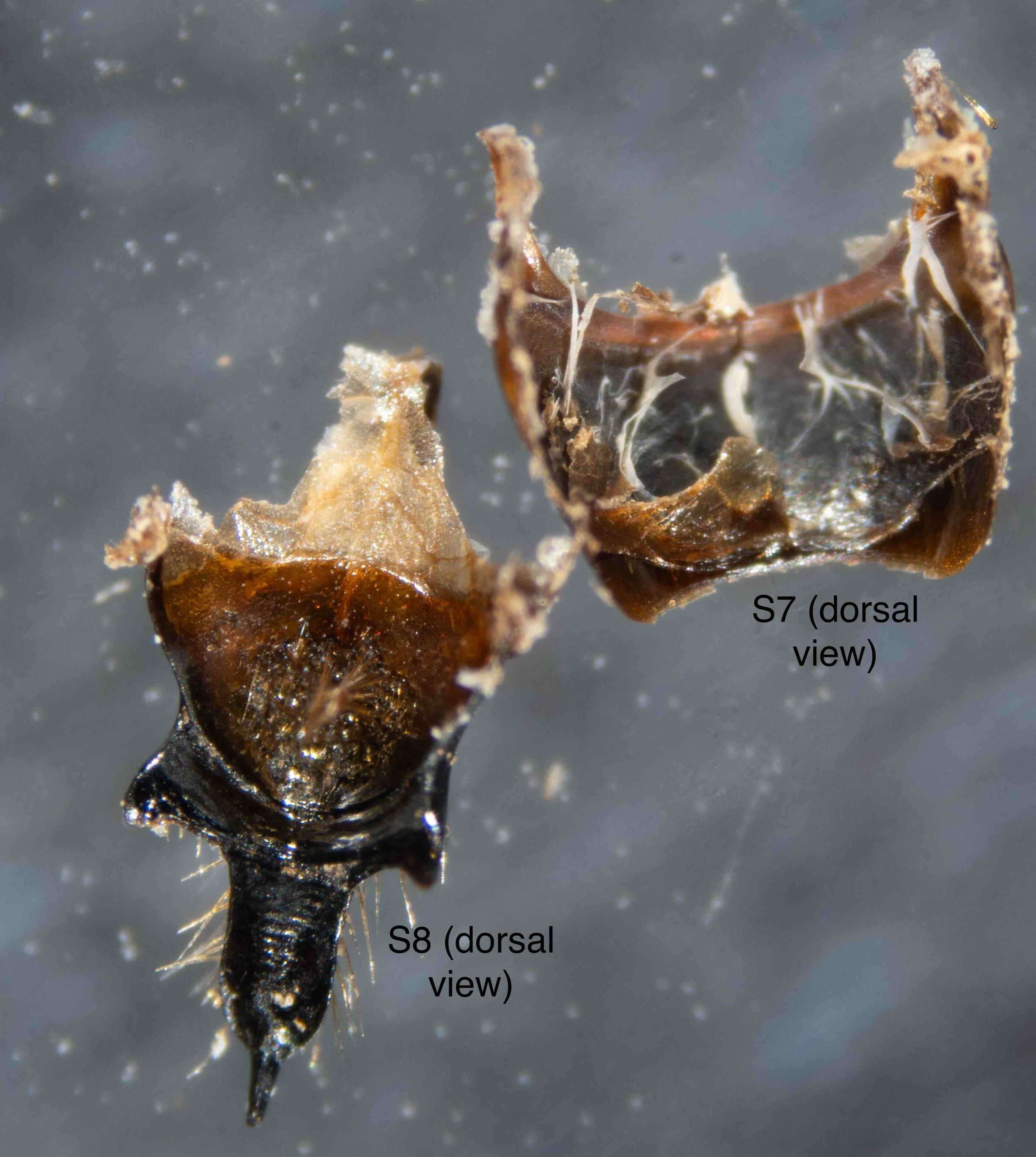
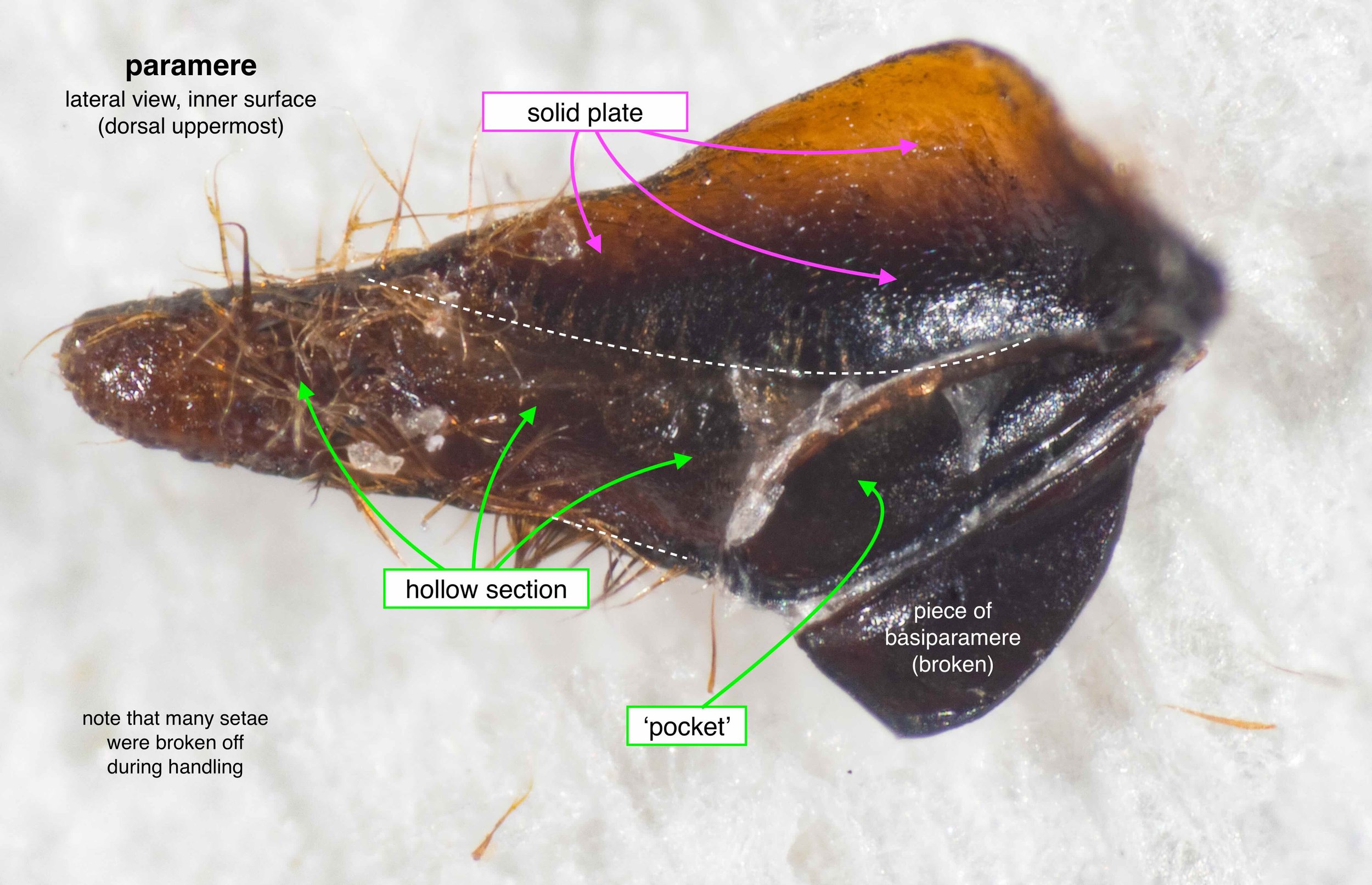
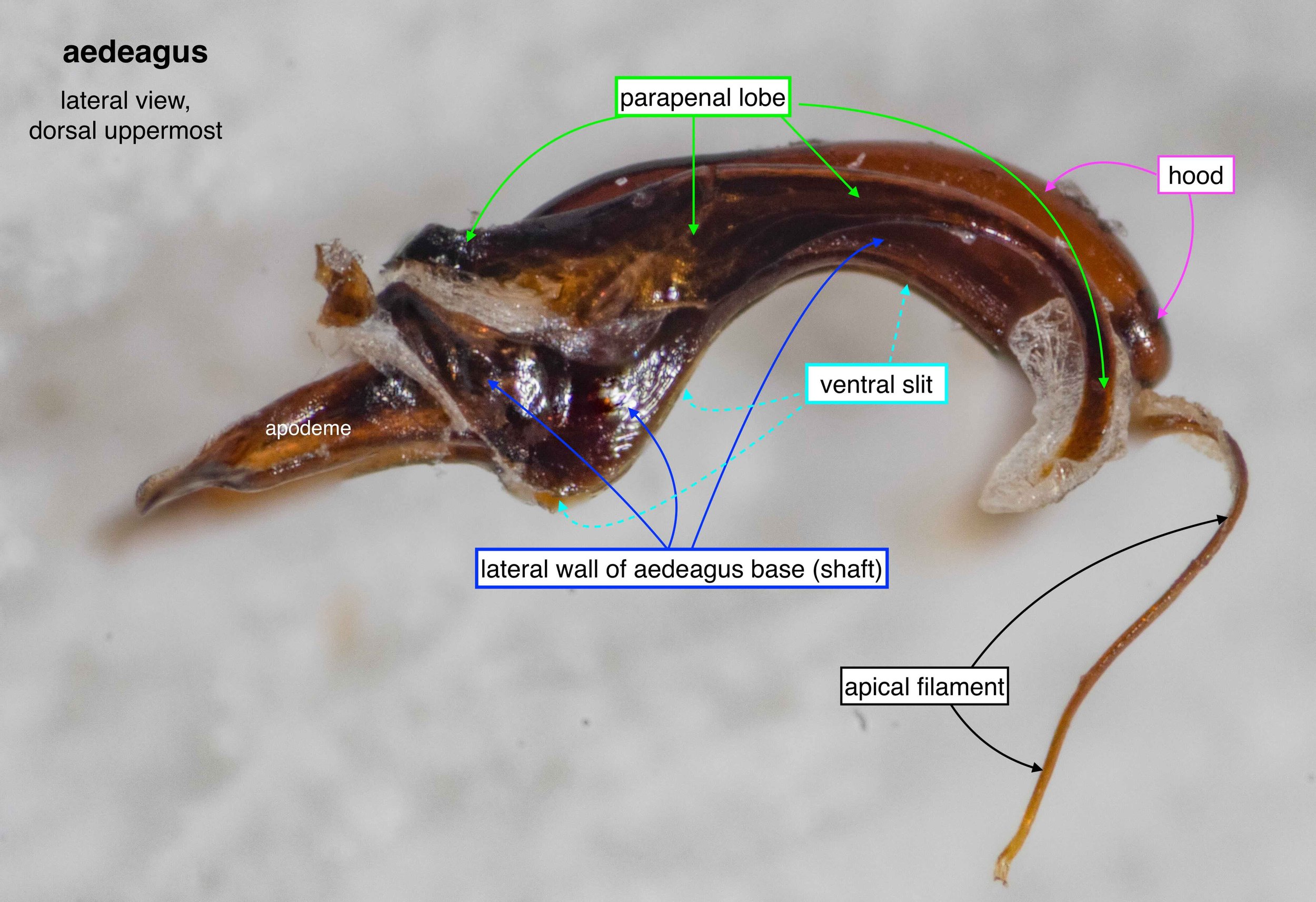
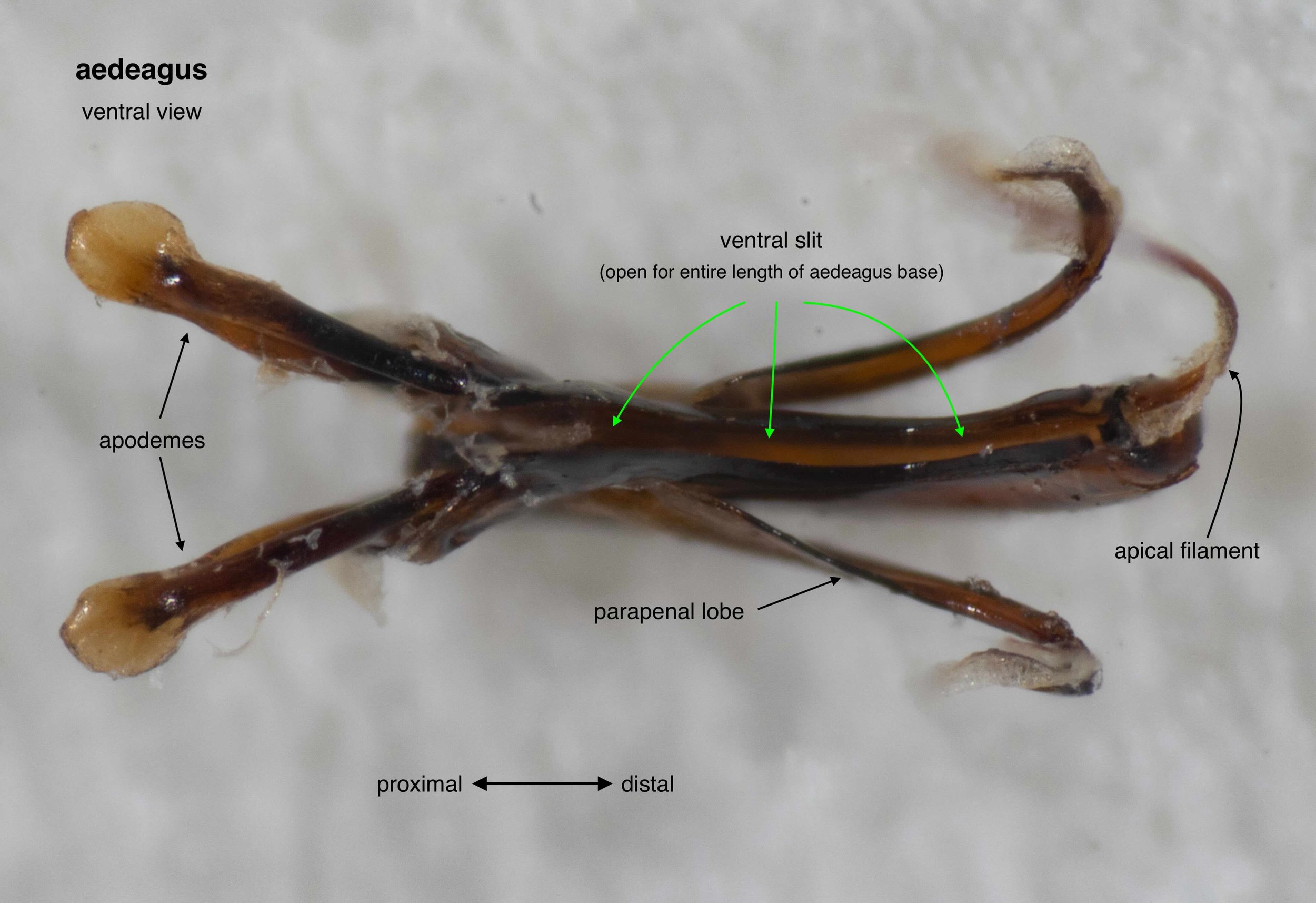
The phallus (genitalia)
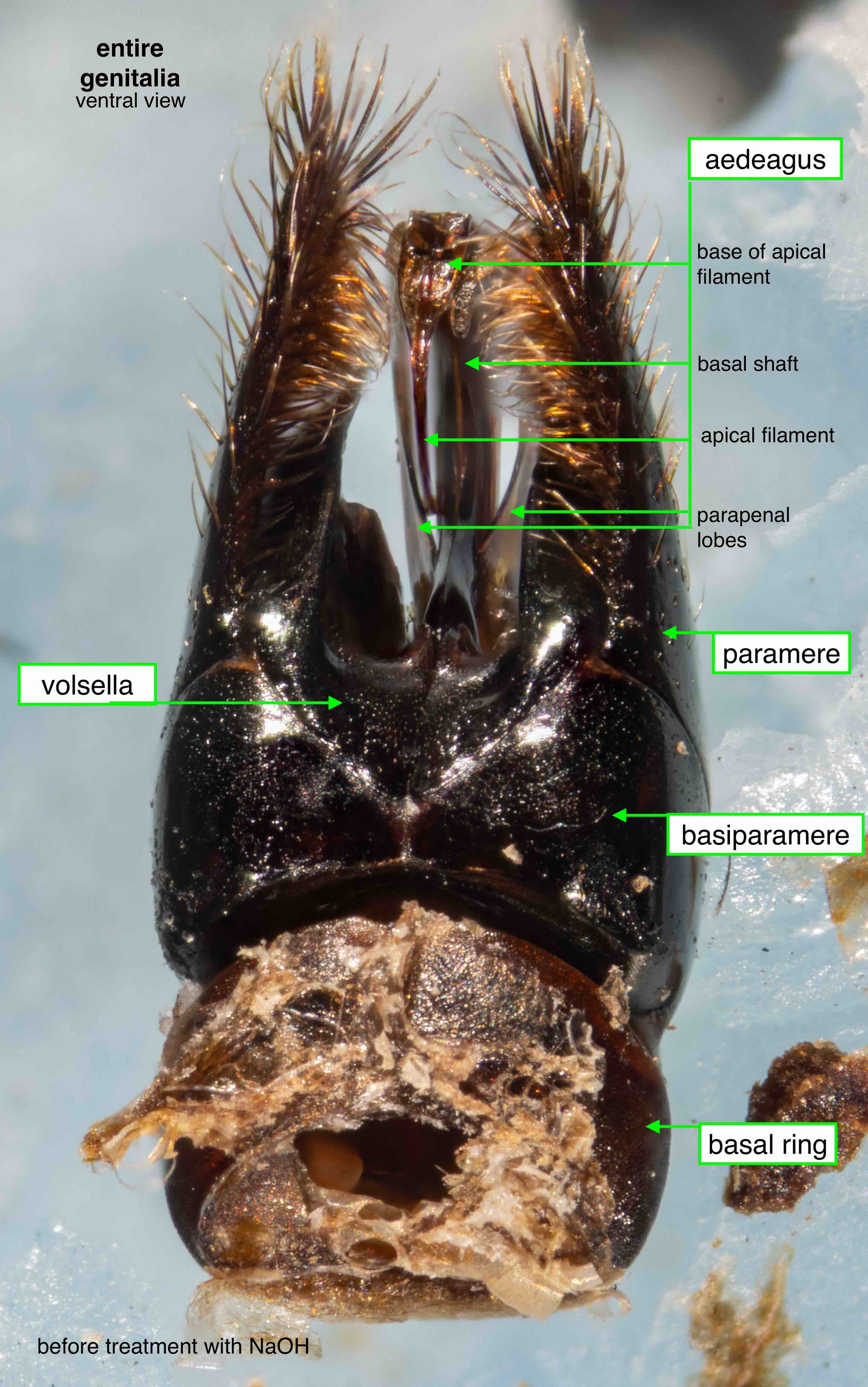
Once isolated, the phallus is revealed as a remarkably beautiful thing. Nature as art, indeed!
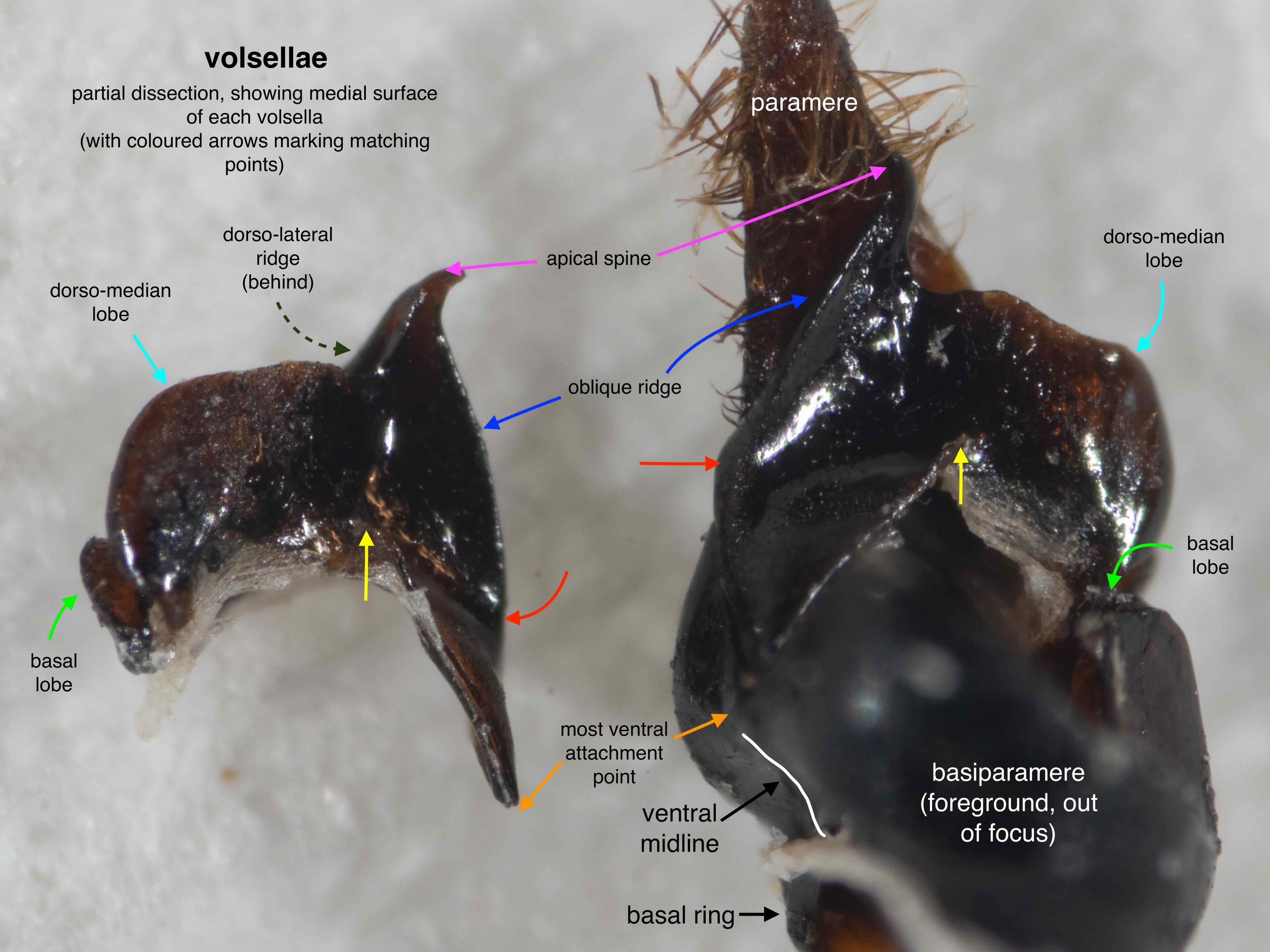
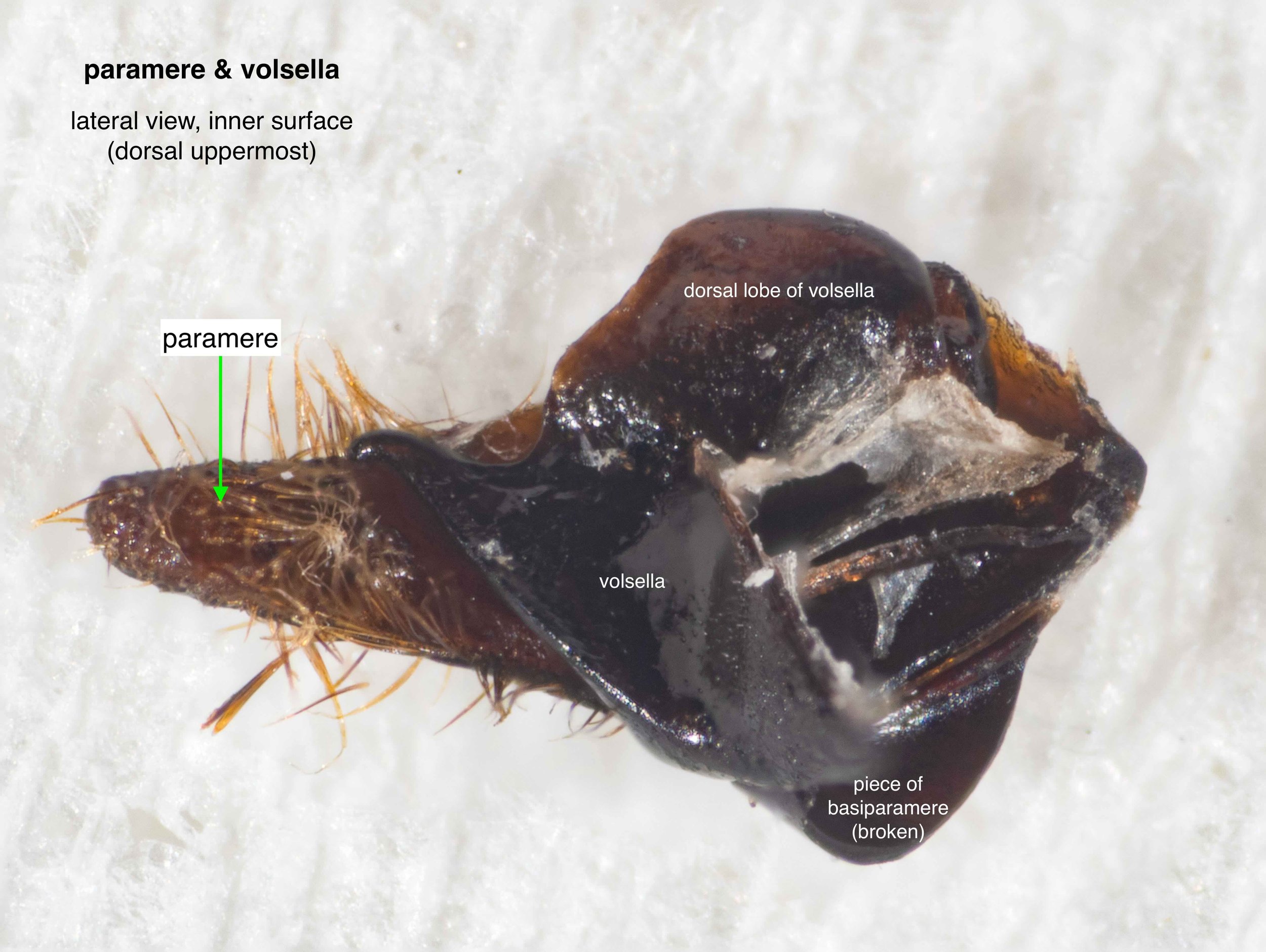
The phallus is comprised of five parts (basal ring, basiparameres, parameres, volsellae, and aedeagus), each with a distinct function. I was surprised, and pleased (!), to discover how readily each sclerite could be identified on the basis of Snodgrass’ (1941) description of hymenopteran genitalia.
The table below summarises what I’ve learned about each of these parts. It is best read in conjunction with the labelled images that follow.
It was possible to separate the individual sclerites of the phallus. The only minor casuality was a break in one basiparamere when I tried to isolate the adjacent paramere. This is unsurprising – the parameres are essentially an apical extension of the basiparameres (Snodgrass, 1941).
Part 3: the twist
In Part 2 I described the flexible nature of the flower wasp coupling apparatus, and briefly mentioned the ability of the male phallus to rotate. But what does this mean, and how does it work?
Brown (2000) provides a detailed description of the process, which I read. And then reread. It’s complicated, and to envisage it I needed to draw myself some pictures.
With this movement in mind, the rounded shape of the phallus base and the deep pocket in the hypopygium make sense. Along with the overlying epipygium, they form the ‘ball and socket joint’ described by Brown (2000).
In describing the male genitalia of Thynninae, and their associated structures (Brown, G.E. 2000, excerpt from p. 211 of Some problems with Australian tiphiid wasps, with special reference to coupling mechanisms).
In describing the hypopygium of male Thynnninae (Brown, G.E. 2000, excerpt from p. 214 of Some problems with Australian tiphiid wasps, with special reference to coupling mechanisms).
Part 4: the action, up close
Now that I have a clearer idea what to look for, I’ve started revisiting my photos. Already I’m learning to identify differences between different species, both in terms of the structure of their terminalia and the manner of their coupling.
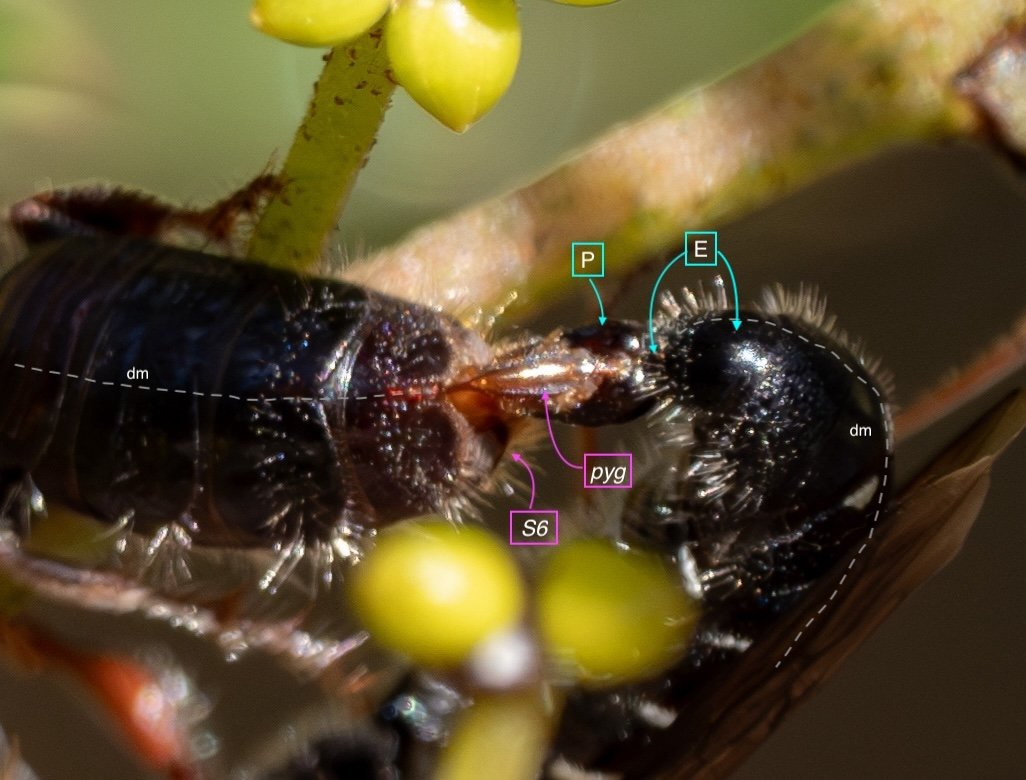
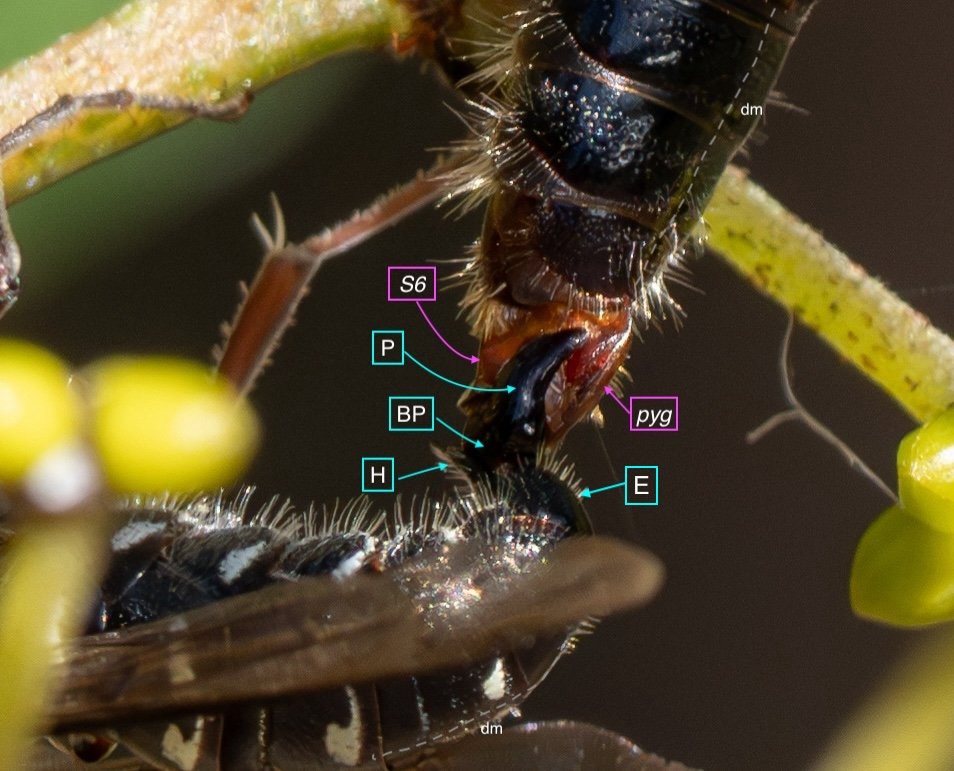
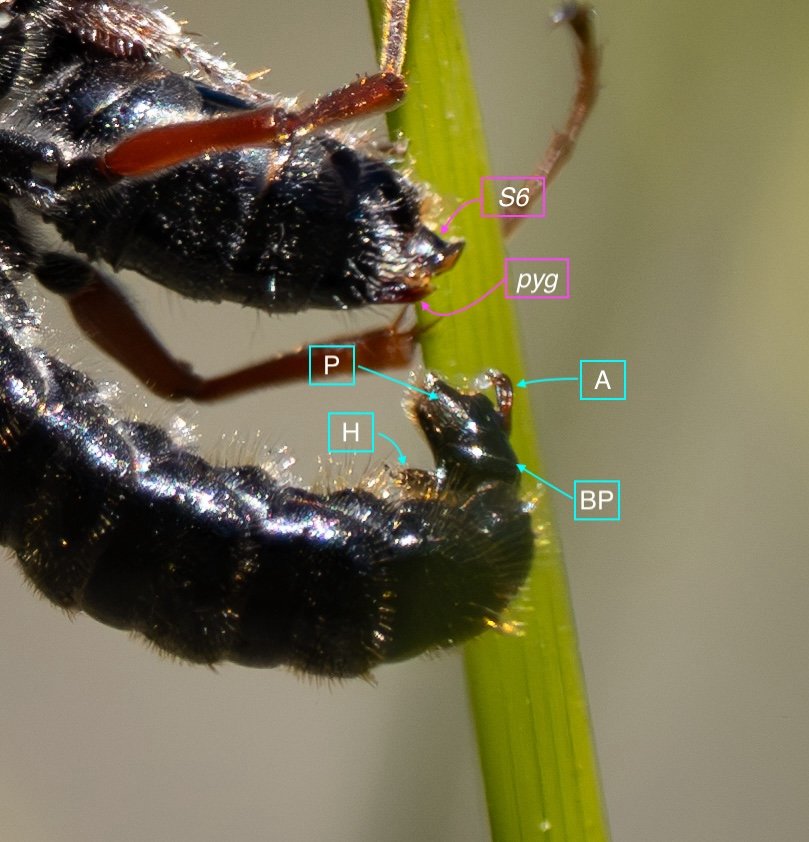
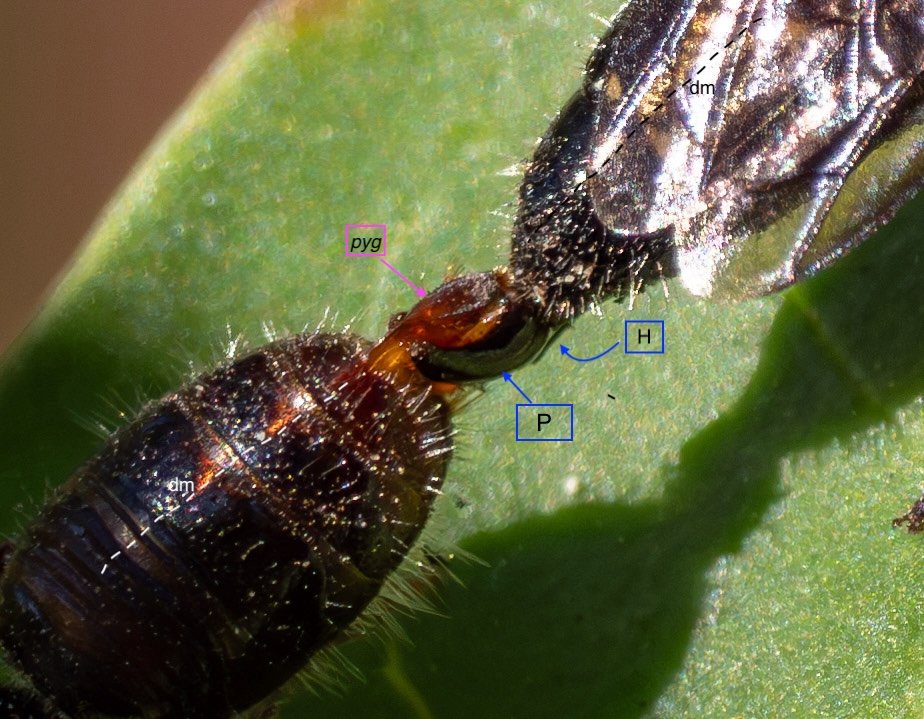
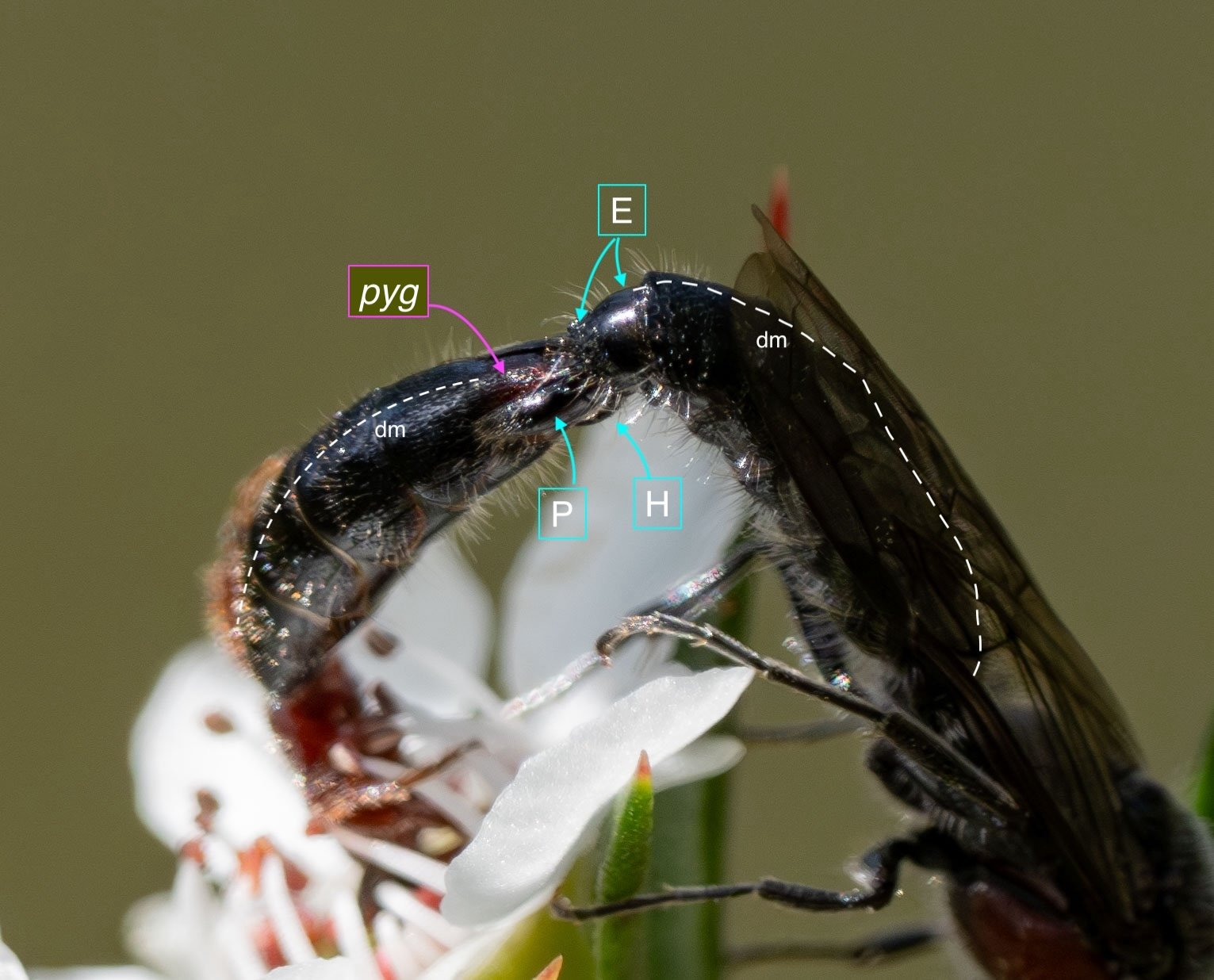
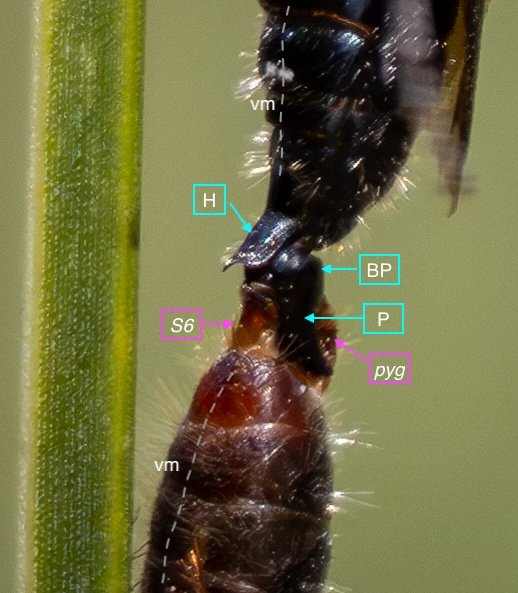
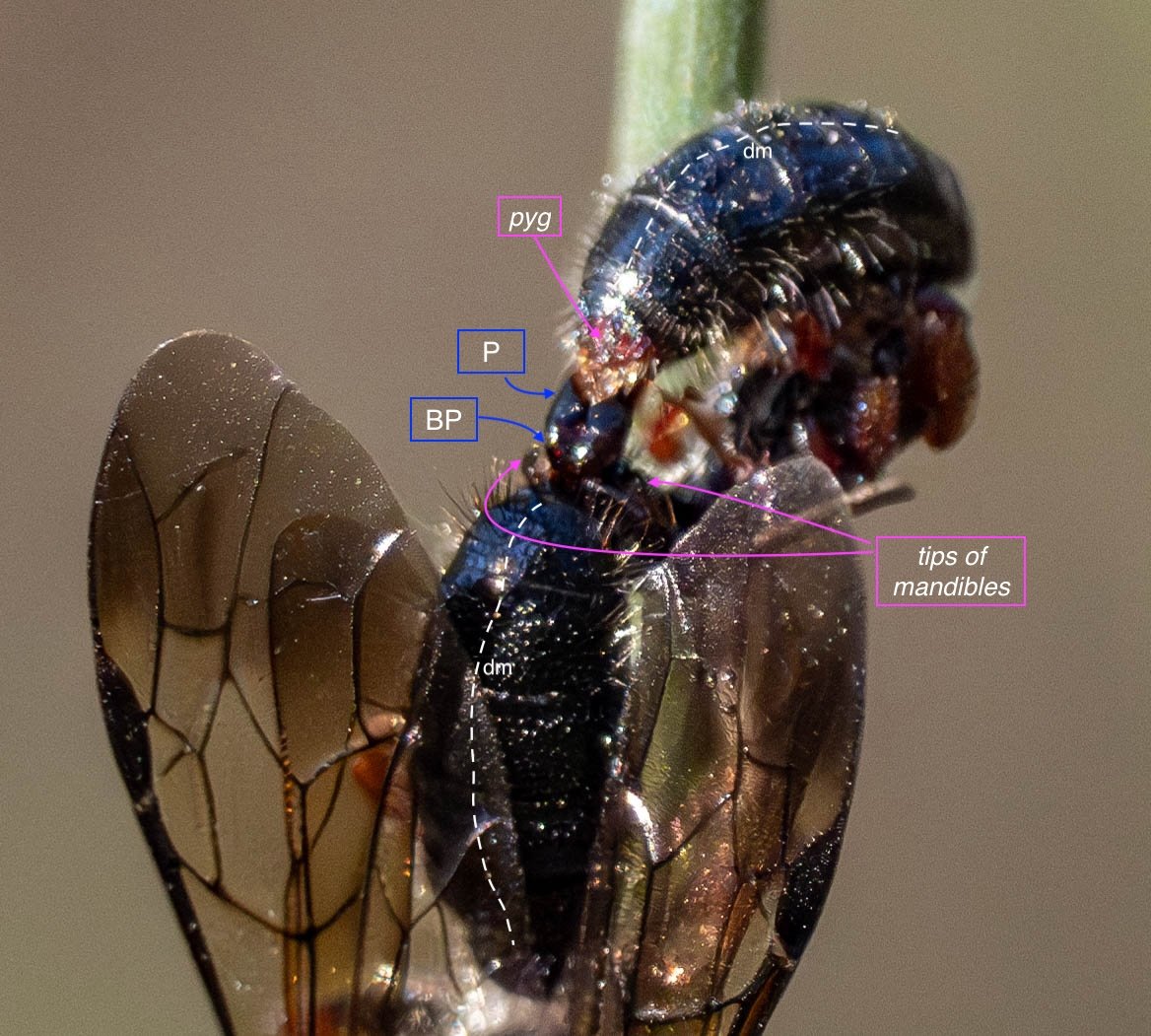
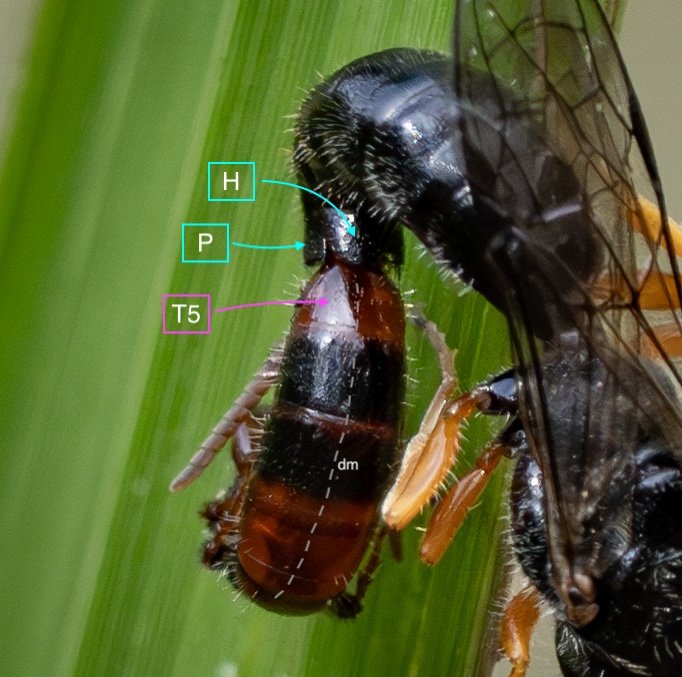
Key: E = epipygium/T7; H = hypopygium/S8; BR = basal ring; BP = basiparamere; P= paramere; V = volsella; A = aedeagus; pyg = pygidium/T6; dm = dorsal midline; vm = ventral midline.
Example 1: securely coupled pair
Example 2: initial coupling
Example 3: the cover page pair
Example 4: Catocheilus (probably)
These colourful flower wasps are the largest we see here. The male’s body is 23mm, the female only slightly smaller. Paul recorded a one minute video of this pair in copula - link to vimeo here.
Example 5: a resting pair
Example 6: same species as Example 5?
Example 7: self-feeding female
This is not the dominant method of feeding for Australian thynnines, although it may be favoured by some genera within the Rhagigasterini (Given, 1954). An additional clue to identity, perhaps.
Example 8: fully exserted
Example 9: a common species here
Example 10: aedeagus on display
Example 11: post copula
Example 12: a very different looking linkage … Eirone perhaps
This pair is quite unlike the flower wasps I’m familiar with. There is no obvious ‘link piece’ between the partners. And they did not do the twist! The simple, rounded hypopygium is suggestive of the relatively primitive genus Eirone (Given, 1958; Semple et al. 2021).
next steps?
This whole adventure began a month ago when I started to consider the identity of our local thynnines. Now that I’ve come to grips with their genitalia, I’m keen to test my collection of photos against published descriptions.
Despite the absence of a comprehensive guide to the 40+ genera of Australian Thynninae, and thanks largely to the efforts of Graham R. Brown, revisions are available for several generic groupings.
A good indoor project for the winter months ahead!
References
Alcock, J. 1981. Notes on the reproductive behavior of some Australian thynnine wasps (Hymenoptera: Tiphiidae). Journal of the Kansas Entomological Society, 54(4), 681-693.
Brown, G.R. 1998. The generic status of Catocheilus Guérom and Hemithynnus Ashmead (Hymenoptera: Tiphiidae: Thynnini). General & Applied Entomology, 28: 89-92.
Brown, G.R. 2000. Some problems with Australian Tiphiid Wasps, with special reference to coupling mechanisms. Hymenoptera: Evolution, Biodiversity and Biological Control (ed. Austin, A. & Dowton, M). CSIRO Publishing.
Brown, G.R. 2001. Status of the Ariphron generic group (Hymonptera: Tiphiidae): a critical review. Australian Journal of Entomology, 40: 23-40.
Dal Pos D., Mikó I., Talamas E.J., Vilhelmsen L., & Sharanowski B.J. 2023. A revised terminology for male genitalia in Hymenoptera (Insecta), with a special emphasis on Ichneumonoidea. PeerJ 11:e15874 DOI 10.7717/peerj.15874
Given, B.B. 1954. Evolutionary trends in the Thynninae (Hymenotera: Tiphiidae) with special reference to feeding habits of Australian species. Transactions of the Royal Entomological Society of London, 105(1): 1-10 + plates.
Given, B. B. 1958. Notes on Australian Thynninae. II The genera Dimorphothynnus, Rhagigaster and Eirone. Proceedings of the Linnean Society of New South Wales 83: 309-26. (open access, via BHL)
Kimsey, L.S. 2004. Illustrated keys to genera of the male wasps in the subfamily Thynninae (Hymenoptera: Tiphiidae). Proceedings of the Entomological Society Washington, 106(3): 571-585
Ridsdill Smith, T.J. 1970. The behaviour of Hemithynnus hyalinatus (Hymenoptera: Tiphiidae), with notes on some other Thynninae. Journal of the Australian Entomological Society, 9: 196-208 (open access)
Semple, T.L., Vidal-García, M., Tatarnic, N.J. & Peakall, R. 2021. Evolution of reproductive structures for in-flight mating in thynnine wasps (Hymenoptera: Thynnidae: Thynninae). Journal of Evolutionary Biology, 34(9), 1406-1422 (open access)
Snodgrass, R. E. 1941. The male genitalia of Hymenoptera. Smithsonian Miscellaneous Collections, 99(14), 1-86 + plates.
In the (most unlikely) event that anyone would like to see more images of the dissection of #2304A, here is a link to my working notes page.