Parasitoids with a difference

Parasitic wasps come in all shapes and sizes.
And they demonstrate a bewildering array of life histories.
The larger or more colourful ones are well known to us. They regularly feature in our photo galleries.
Yet for every species we see, there are probably hundreds we don’t. Most wasps are tiny – and more challenging to find or photograph.
And then there are the surprise packets!
It’s not uncommon for our up-close studies of insect life cycles to be thwarted by parasitoid wasps. These discoveries are always exciting. Unlike most field sightings, we have both sides of the story – the parasite AND the host! Such surprises always warrant a blog on our website.
This week revealed yet another surprise.
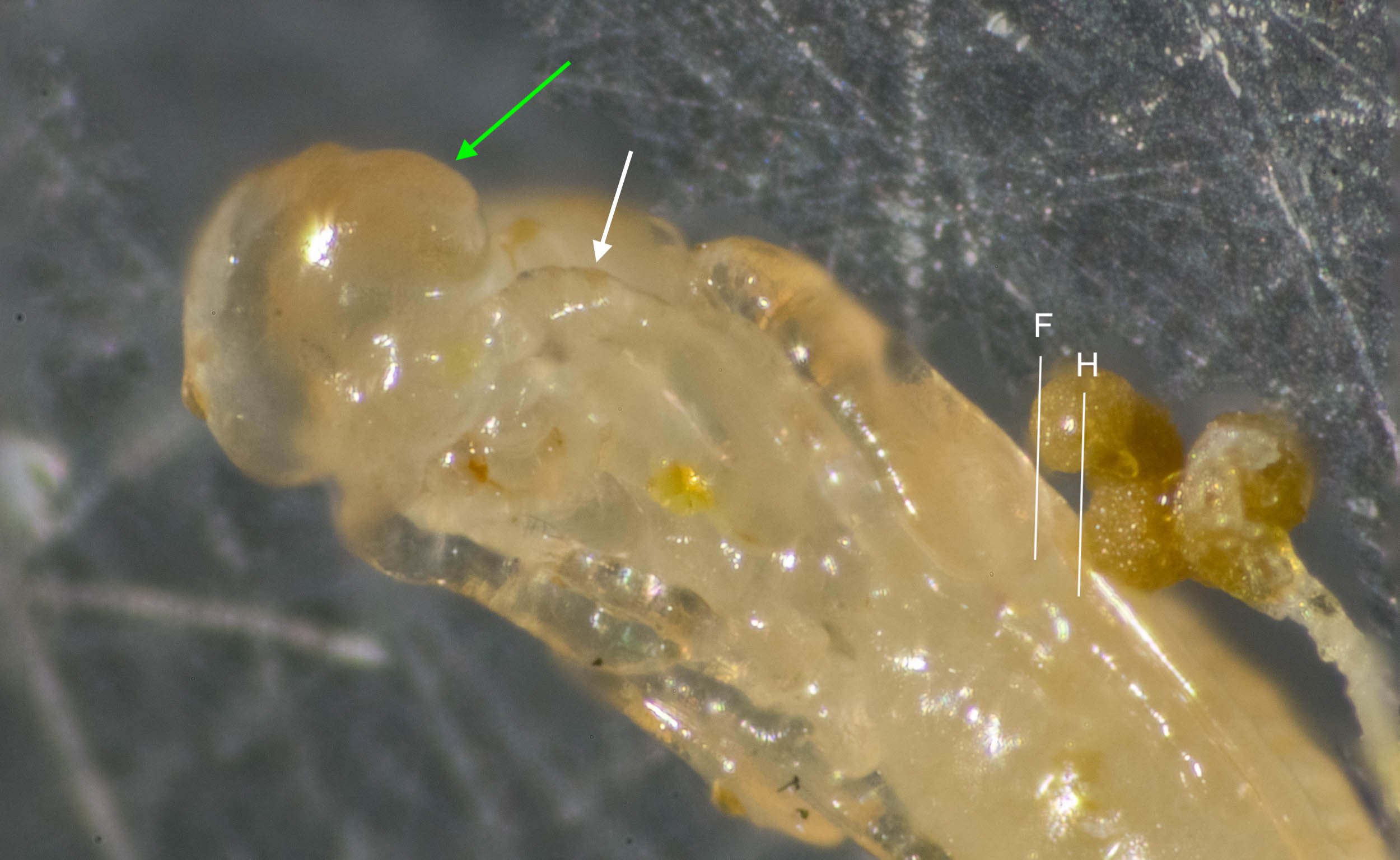
Inside the thick-walled mud nest of a crabronid, where I expect to find a single larva feeding or pupating safely inside the further protection of a cocoon, I find this – a mass of yellow grubs, along with dozens of tiny adult wasps.
This is a wasp quite unlike any we’ve encountered before. In contrast to the various examples above, this species is a gregarious (not solitary) ectoparasite. I was sufficiently fascinated to drop my current crabronid project and take another plunge into the life of parasitoid wasps.
The backstory
Throughout January and February we watched this female Pison come and go. She would ferry loads of mud (as here) to construct her nest cell, stocking each completed chamber (‘cell’) with paralysed spiders and a single egg, before progressing to build the next cell. Unfortunately, she chose a vulnerable location … amongst our outdoor furniture. I accidentally destroyed her nest on 28th February.
It all started in late February. Having accidentally broken open the mud walls of a Pison nest (again!), I gathered a few pieces for study. One chunk of intact mud nest and one exposed cocoon. I stored them together in a close container … and waited.
I was hoping to collect the emergent Pison and so try for species level ID. Or perhaps a metallic cuckoo wasp or thick-skinned velvet ant …
Instead, this week I discovered about a dozen tiny winged insects crawling about the dish.
Melittobia australica (Chalcidoidea: EULOPHIDAE). They are probably here in large numbers, and having a significant impact on populations of various bees and wasps.
At this point I normally embark upon the long journey of species identification. Careful examination of specimens, photo-matching with images online, searching for and studying the literature. Putting a name to a face often takes weeks.
Identification was quick & easy (for a change)
This time it was just too easy! A quick web search for 'Pison parasitoid' and I struck gold. I was merely hoping for a hint, perhaps a family or subfamily ... but almost immediately I had an identification. Melittobia … and there is just one species in Australia. Another quick check and it seems a good match to detailed descriptions of the species (Dahms 1984a).
Melittobia australica is common, well-studied, and somewhat infamous. These tiny Australians have spread to many parts of the world and have adapted well to their new environments. A single wasp can found a population of thousands in just a few months, and they are not at all fussy about their hosts. Melittobia parasitise many species of solitary bees and wasps, and even the thick walls of mud nests are readily breached.
It is the intricate life cycle of Melittobia that fascinates researchers. As Matthews and colleagues (2009) explain:
Melittobia … "exhibit simple forms of many social insect traits, including overlapping adult generations, different female phenotypes, close kinship ties, parental care, and altruistric cooperative escape behaviours". (p. 251).
Indeed, many school students in the US know Melittobia well. WOWbugs, Melittobia digitata, are available as kits for use in life science classes.
Still, they're new to me. And intriguing!
Where did they all come from?
And what are they up to now?
After poring over comprehensive reviews of Melittobia (Dahms 1984b; Matthews et al. 2007), I have answers to these questions.
The following description of events is hypothetical and generalised, but possible. Arguably, it is even probable. In any case, the whole endeavour has taught me much I didn’t know about these ‘polyphagous, gregarious idiobionts’. Biology jargon aside, here’s a tale of who, how, when and where.
1. Breaking into the mud cell
In late February, a female Melittobia broke into a cell of the Pison mud nest. She had a sister with her. Working together they chewed their way through the mud wall. She had already mated with one of her brothers before leaving her own birthplace, and so was carrying the sperm she’d later need to fertilise her eggs. Her wings were long, her abdomen small. She could fly, although not far without assistance. She was born in a nearby spider wasp nest, but she’s not fussy in her own host selection. Any above-ground, enclosed nest of a solitary bee or wasp will do. Pison suits her needs admirably.
2. The wait
Within the cell was a single, large Pison larva. It had already consumed most of the paralysed spiders provided by its mother, but was still feeding. The invading female Melittobia watched and waited from the edge of the chamber. Occasionally she would stab the larva with her stinger, then turn about and drink the leaking body fluids. She didn’t need much, as she could survive weeks without feeding.
By the end of February, the Pison larva had constructed its cocoon and was ready to pupate. It did not get a chance.
3. Entering the cocoon, and now a proper feed
The foundress makes a hole in the papery cocoon with ease. It is thin and she has impressive mandibles. Over the next two days she and her sister take repeated liquid meals from their quiescent host.
Now well fed and rested, it is time to prepare the host to receive her eggs. She doesn’t want it to move and risk dislodging her or her eggs once she starts to lay. This time she stabs deeper with her ovipositor and injects a dose of venom (Deyrup et al. 2006). The host is paralysed, its development arrested. It remains alive … for now. It is early March.
4. Egg laying begins
Nourished by her fluid meals, the little wasp’s ovaries are activated. Her eggs mature and she starts to lay. There is no need to inject the eggs inside the host body - the cell and cocoon provide all the shelter they need. She simply releases the eggs onto the host’s body. They adhere to the cuticle and to each other, forming clumps in the intersegmental grooves of the prepupal Pison. Further along the body, her sister is doing the same.
For the next few weeks she continues to lay, occasionally taking a fluid meal. Between them, the sisters produce hundreds of relatively large eggs.
5. Hungry, growing larvae
The eggs hatch in a few days and the small grubs start to feed. For a while the host simply loses body fluids, but the later-born larvae help themselves to more solid meals. The parasites feed and grow, moulting when necessary. They retain the waste material within their bodies, ejecting it only when they are ready to pupate. In the cool of early Autumn, they take several weeks to pass through the larval stages. When they are ready to pupate, they roll away from the host, void the waste they have retained in their bodies, and begin their own transformation to adult wasps. But all the while, younger siblings and cousins are hatching and taking their place to devour the collapsing body of the host.
6. The first-born daughters
The first eggs laid develop rapidly, and they’re quite unlike their mother. Most are fat-bodied females, filled with well-developed eggs. Their wings are short and in contrast to their travelling mother, they have no intention of leaving home. They mate with their brothers and soon join their mother in laying clumps of eggs upon the host. Relative to Melittobia, the host body is massive. Plenty of food for all.
7. Sons and grandsons of the foundress
Males are few and distinctive. They develop from unfertilised eggs, typically produced when their mother depletes her supply of stored sperm. Males are all blind, flightless and short-lived. When receptive females are in short supply, they will fight other males – sometimes to the death. They release pheromones to attract virgin females and then begins a period of complex courtship. Brothers mate with sisters or any other available females … including an aunt or mother, at need.
8. Fully winged daughters leave home
Most of the larvae develop into long-winged females. Once a male becomes available, they mate and then seek to leave their natal nest. Some clearly succeeded … they were the ones wandering about the dish this week. And some were clearly successful in finding a new host! The eggs and young larvae on cocoon provide proof.
The contents of the Pison cells, as uncovered on 7th May after I tore open the two cocoons.
Cell A snapshots
The cocoon within cell A contained hundreds of wasps across a wide range of developmental stages. The foundress and her first-born, reproductive daughters were obviously highly productive! The result is a very large family, the siblings born over many weeks. (All images were taken 7-10th May).
Cocoon B snapshots
The larva in cocoon B was parasitised by females dispersing from Cell A … and just days before I first noticed them on 7th May. The transformation has been rapid, and the drama continues.
Some known unknowns
Might there have been just a single foundress? Yes. The mass of larvae in cell B could well be the offspring of just the founder and her eldest daughters.
Could she have entered the cell after I collected the nest? Unlikely. The container was sealed, it is hard plastic, and I can find no sign of chew holes in it.
How long did it take from egg to adult? Dahms (1984b) reports a total of just 14-18 days, but that was at temperatures of 25-30 degrees C. It is much cooler here, and they were housed at around 18 degrees. Development is acknowledged as being temperature dependent … I took a guess at 4-5 weeks. Indeed, 37 days at 20 degrees has been reported (Freeman & Ittyeipe 1993). Over coming weeks I hope to provide a more accurate measure for our local wasps and conditions. I’m keeping a close eye on the contents of cocoon B!
Is the original foundress among the wasps I see now? No. Once mated, Melittobia females typically live for around five weeks and lay most of their eggs within the first 15 days (Freeman & Ittyeipe 1993). They can delay the start of oviposition and feeding for several weeks (as in my hypothetical). Our founding female must have commenced adult life in late February. She would not have survived past mid April.
UPDATE, 4 months on
Throughout winter, both larval masses lay quiescent. I checked in on them regularly, looking for signs of either decay or development. Nothing. Until spring …
References cited
Dahms, E.C. 1984(a). Revision of the genus Melittobia (Chalcidoidea; Eulophidae) with the description of seven new species. Memoirs of the Queensland Museum 21(2): 271-336
Dahms, E.C. 1984(b). A review of the biology of species in the genus Melittobia (Hymenoptera: Eulophidae) with interpretations and additions using observations on Melittobia australica. Memoirs of the Queensland Museum 21(2): 337-60 (open access)
Deyrup, L.D., Rivers, D.B. & Matthews, R.W. 2006. Venom from the ectoparasitic wasp Melittobia digitata (Hymenoptera: Eulophidae) induces paralysis and developmental delay in natural and factitious hosts. Entomological Society of America 99(6): 1199-1205 (available via ResearchGate)
Freeman, B.E. & Ittyeipe, K. 1993. The natural dynamics of the eulophid parasitoid Melittobia australica. Ecological Entomology 18: 129-140.
Matthews, R.W., González, J.M., Matthews, J.R. & Deyrup, L.D. 2009. Biology of the parasitoid Melittobia (Hymenoptera: Eulophidae). Annual Review of Entomology, 54: 251-66 (available via ResearchGate).
Other reading
Quickie, D.L.J. 2015. Idiobionts, kionobionts and other life history traits. Chapter 6 in The Braconid and Ichneumonid Parasitoid Wasps: Biology, Systematics, Evolution and Ecology. Wiley Blackwell. pp 87-105