Nesting habits of a little-known wasp

It is always exciting to discover an insect or spider that we’ve never seen before. Questions are raised. What is it? How does it feed? Where does it breed? And how has it escaped our notice until now?!?
The latest discovery: tiny black wasps.
I probably would have overlooked them – except that in early September there were dozens in one small area, performing a ground-hugging, dancing flight. Curiosity piqued, I set to investigate.
More than a week of field work and as many days in the lab and at the computer … and we are now delighted to share the story.
Brand new insights into the life and times of a rarely seen species.
The early sightings
The site is a sunny, bare patch of flat ground. Coarse grains of sand overlie a firmly packed base of sandy soil. An ever changing pattern of fallen leaves, twigs and eucalyptus fruit decorate the surface. This is one of our most frequently used tracks, but since first sighting the wasps last week I’m loath to set foot. Instead, I perch nearby and watch closely.
The wasps are small and difficult to spot (see green arrow), except when in flight. I scan the field, alert to their bobbing motion, then track them until they either land or disappear down a hole.
It seems we caught the early action. My first sighting was a single, nesting female on 1st September. A week later numbers had grown and the sandy patch was attracting many females looking for nest sites, along with numerous cruising males. Then, just a few days on, yet another change. By 11th September, most of the wasps were females delivering prey to established nests. There was an occasional visiting male, but very few. There are now at least twenty nests within a few square metres.
Part 1: Field study
Typically after sighting something unusual my first step is to concentrate on species identification. This demanded a different approach. After a quick check of the literature I assumed the wasps were a species of Rhopalum, a type of crabronid, but my first priority was simply to watch. I would come back to identification later. Breeding events are often short-lived and dynamics at the nesting site were obviously changing fast.
So for nearly two weeks I just observed and photographed the little wasps as they came and went. From my previous bouts of obsession with sand-nesting wasps, I had some idea what to look out for. For example …
What are they preying upon, and how do they carry their catch? Podagritus prey upon large flies (see Digger wasps, Dec 2020), and Rhopalum is reportedly a fly catcher too.
Do females share nest burrows? Some sand-nesting wasps seem to collaborate, such as Cerceris, with one standing guard while others hunt.
Are their burrows hidden? In one small patch of sandy soil last year, I watched Sphodrotes carrying shield bugs into open nests that were hidden away under fallen leaves. Nearby, a larger wasp (Austrogorytes) had a highly visible mound but she would cover the nest opening each time she departed to hunt for leafhoppers (see More wasp diggings, Jan 2021).
NESTING BEHAVIOUR
After many hours spent peering at this unpromising-looking sand patch, here’s what I can now say about the nesting behaviour of this species.
They prey on flies – various types and a range of sizes
The photos below tell the story. From these shots alone we can say that the prey includes long-bodied crane flies (Tipulidae) and stout little signal flies (Platystomatidae).
It did take us a while to recognise the crane flies … until we realised that their legs had been removed! Crane flies have characteristically long legs so it seems that the wasps simplify transport by first cutting them off. That’s not unprecedented wasp behaviour. For example, some spider wasps (Pompilidae) amputate the legs of huntsman spiders before dragging the still-living victims back to the nests.
And it seems safe to assume that the flies our little black wasps are carrying are indeed paralysed, not dead. This is the common strategy used by many kinds of predatory wasps, including the mud-nesting wasps I was fascinated by last summer (see: A window into mud-nests, Dec 2021; Wasp forensics, May 2022).
Some burrows are in the open, others are under leaves
Many burrows were exposed, while others were hidden beneath fallen leaves. But leaves move with the wind, so even if the wasps seek to hide their nests they may not stay that way for long.
Which raises another question. How does a female find her burrow? I assume that they use landmarks, such as stones, sticks and fallen leaves. If so, there must be considerable redundancy in the system. The wind regularly displaces the leaf litter, so much so lately that I have trouble keeping track of nest locations. Indeed, the behaviour of incoming wasps suggest that they progressively narrow their search radius, perhaps triangulating between multiple landmarks. It is common to see a returning female, burdened with prey, circling the area several times before entering her nest. Those with an exposed nest dive right in, while those with a hidden entrance land and then quickly dash beneath the leaf.
They are ‘home alone’
I’ve only ever seen a single female using a nest burrow. Of course, I can’t identify individuals so I rely on less direct evidence. That is, I have never seen a second wasp enter a nest where I know one has already entered. And after the owner has left, I have looked for a second wasp ‘on guard’ or even departing the nest, but never sighted one. And I have been looking!
Activity is highest in the middle of the day
I quickly established that the most productive time for photography was the few hours around midday. Before about 10am, after 3pm, or on cold and cloudy days, the wasps were nowhere to be seen. I assume that the females remain within their burrows until warmed by the sun. No complaints from me! Spring days are still quite chilly here on the far south coast.
Burrows are left open by day, but closed at night
The females leave the burrows open during work hours. They simply take a quick look out the door, then fly off to hunt. This means that they can quickly disappear from sight on their return … at least, once they find their nest. But when I check in the late afternoon, the doors are all closed. Sand or small pebbles block each hole and quite effectively disguise the nests. Sure enough, when I return at around 9am the next day, still closed – and by 10am they’re wide open, and stay that way until mid afternoon.
A closed burrow must provide a measure of safety for the resting female, her food cache and her offspring. Shelter from the rain, some insulation from the cold, and a barrier to nocturnal hunters, scavengers and parasites.
NEAR NEIGHBOURS & PASSING TRAFFIC
The apparently bare patch of sand is certainly not vacant land. As Paul and I have been peering intently at the little black wasps, we’ve been distracted by a parade of spiders, ants, bees, bugs and even another type of sand-nesting wasp. Our little black wasps have moved into quite a busy neighbourhood!
OCCUPATIONAL HAZARDS
For such diminutive insects, these wasps are remarkably successful hunters. A single female might collect a dozen or more flies in one day. I recorded one wasp delivering four flies into her nest within 100 minutes.
But things don’t always go to plan. Here are three examples of the impact neighbourhood conflict can have on productivity.
Crafty wolf spiders
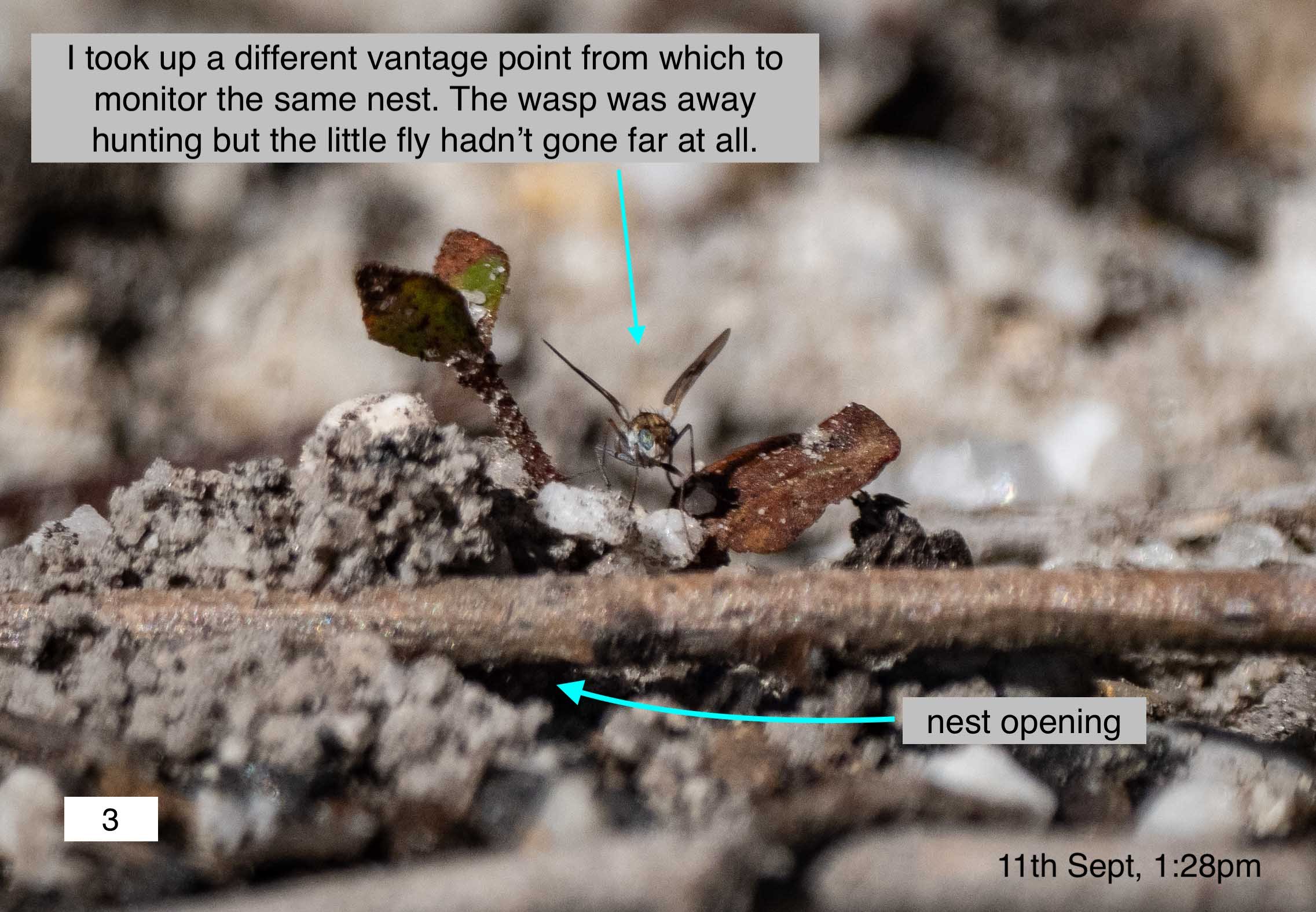
They may be just 5mm long, but these wolf spiders are clever hunters. During the middle of the day, when the wasps are most active, they are on the prowl. The clever part is the way they target the nesting wasps. They stake out the area around the nest, clearly alert for the incoming females. I saw this play out repeatedly. Different spiders, different nests. Sometimes the spider would hide under a nearby leaf, darting out when the wasp came in to land.
The wasps are alert to the danger … as the following series shows. In this case the wasp was in the early stages of nest construction. The spider is clearly after the wasp, not her prey, as she is not carrying any.
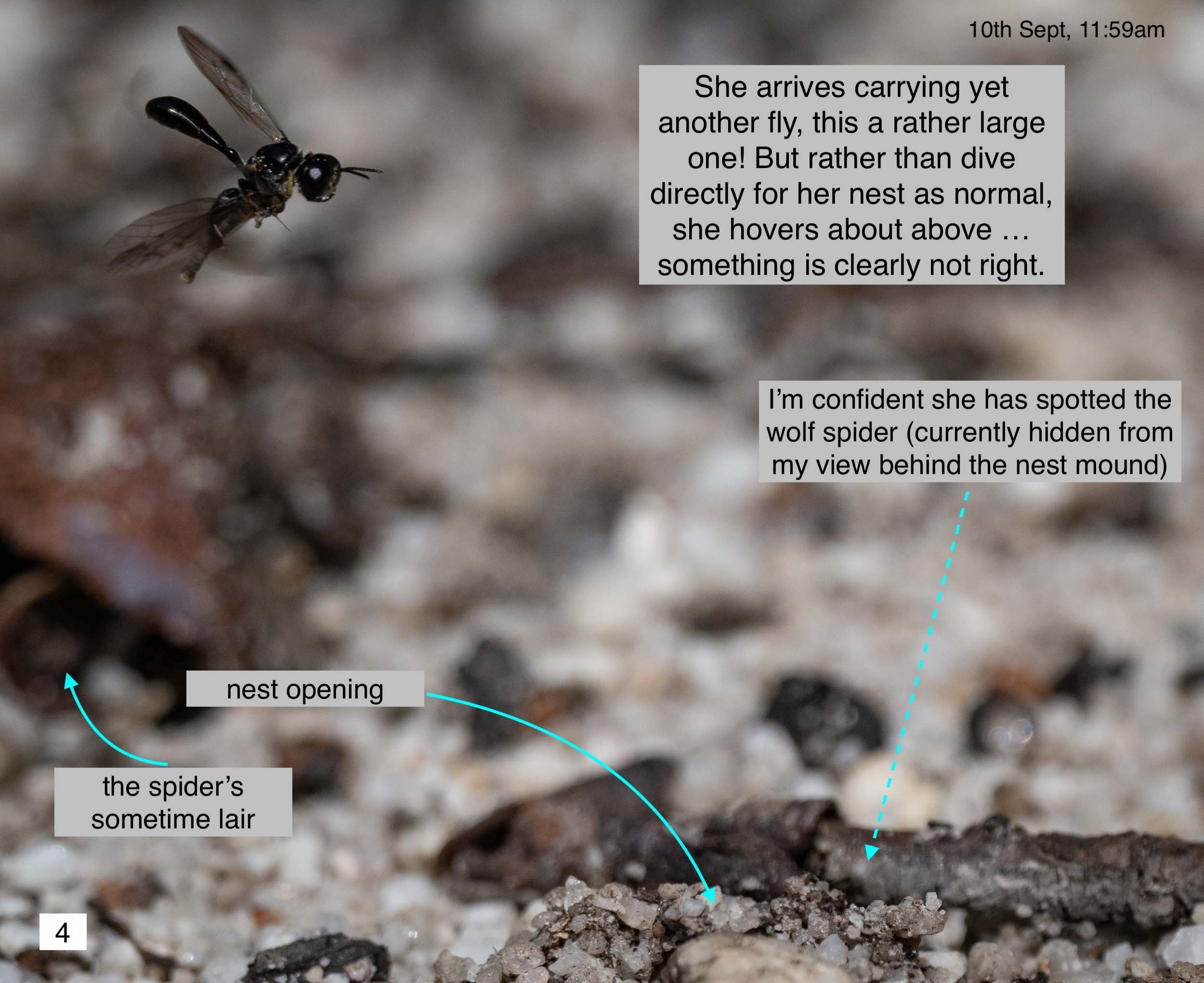
This was no isolated occurrence. Another day, another wasp, and another wolf spider.
This time I noticed a spider hiding a short distance from a nest. It was moving in and out of its lair, clearly reacting to the brief appearances made by the wasp. After two successful deliveries, the wasp returned with a third (large!) catch … and the spider leapt! It missed its shot, and I did too – but I witnessed the action. The elusive wasp flew off, still clutching her prey. She returned within minutes, surveyed the scene –which included an emboldened spider sitting out in the open – and again flew away. It was another 20 minutes before she returned and warily entered her burrow.
Thieving ants
I noticed another of the nesting wasps facing a different challenge. She landed at her burrow, carrying prey, but it soon became clear that something was amiss. Rather than dive into the burrow, she began to dig. The entrance to the burrow was blocked by sand and small stones. Perhaps the mound had been disturbed while she was out hunting. It was an exceptionally windy day and maybe sticks being blown along the ground had done the damage. Or perhaps she simply went hunting without first completing her burrow.
Whatever the cause of her problem, she was clearly agitated and almost frantic in her excavations. Putting her cargo aside, she set to work … and therein lay the problem. Free food for wandering opportunists!
Sneaky flies
Amid the various other insects and spiders using the site, the actions of one type of fly particularly fascinated me. These small, silver-bodied, blue-eyed flies sneak around the nest mounds. When away from the mounds they strut about, flicking their wings. Yet they seem drawn to active mounds, where they stand motionless – sometimes in the open, at other times half-hidden by leaves or sticks. They are definitely up to something!
The wasps seem a little bothered by the flies, but not enough to be driven away from their nests.
My first guess is that the flies are parasites, looking to hijack a well-stocked burrow to house and feed their own larvae. Perhaps the fly dashes in when the wasp is out … although I have seen no evidence of that. Or they may even be quick enough to attach an egg to the paralysed fly as the laden-wasp arrives at her burrow. Seriously, such things happen! Flies are rather notorious kleptoparasites, and have evolved all sorts of bizarre behaviour to capitalise on the hard work of others (Sivinski et al. 1999).
At this stage all I know for sure is that the flies are attracted to the nests, and the wasps aren’t too thrilled. To help solve the puzzle I need to catch one of these little flies. Accurate identification may help explain just what they are doing hanging about the nesting wasps.
... and then there are the elements to contend with!
I happened to be on hand when a sudden wind gust scattered fallen leaves across the surface … and completely levelled one of the nest mounds I have been monitoring! The nest was active only moments before but the female was off hunting at the time her nest was flattened.
My first thought: “Oh, I was right! Windblown leaves can indeed lead to nest collapse.”
My second thought: “What will she do when she returns? Dig it open, like the star in the ‘thieving ants’ drama described above?”
Within seconds, one of those sneaky flies was on the scene and clearly excited. Soon after that the homeowner returned, thoroughly confused. She hovered nearby, landed briefly, then flew about the site checking around other leaves as if hoping she had simply lost her bearings and would find her nest eventually. It must have gradually become clear that this was indeed the right spot … she kept returning, landing, and scurrying about on the sand.
However, she did no digging in the rubble during the time I watched on. Eventually I lost sight of her and the demolition site now appears deserted.
Part 2: Back in the lab
So with the field data in hand, it was time for some serious research. Just who are these wasps? What is already known about them? And is there anything we have observed that might add to that knowledge?
PRELIMINARY ID
In between bouts of kneeling in the dirt wasp-watching, I had already begun combing the literature. Hazarding a guess that the wasps are Rhopalum, I searched for information on their nesting behaviour. There is surprisingly little published on Australian Crabronini but I quickly discovered one very relevant paper.
It was (my heroes) Howard E. Evans and Robert W. Matthews to the rescue yet again! In their 1970 study of sand-nesting wasps near Canberra, they discovered a single Rhopalum variitarse female stocking her underground nest with tiny flies (Evans & Matthews 1971a).
That’s a good match! Could this be my species?
Extract from page 3 of Evans, H.E. & Matthews, R.W. 1971a. The ‘preceeding’ species the authors refer to is Podagritus leptospermi.
First question: is my wasp a Rhopalum?
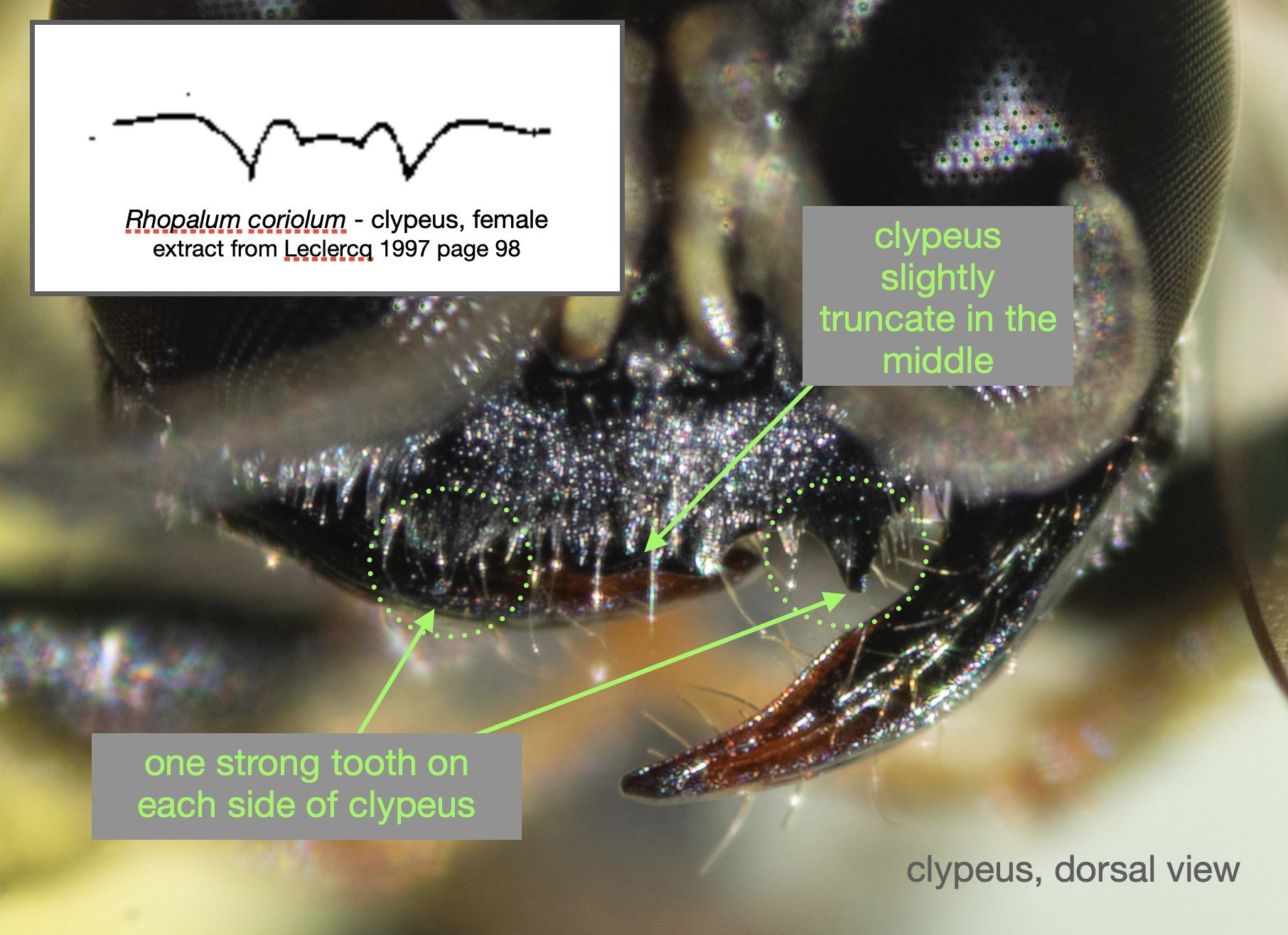
Both Rhopalum and Podagritus belong to the tribe Crabronini and it turns out that distinguishing the two is not entirely straightforward. In fact, they are so alike that some authors have suggested collapsing the two genera into one (Bohart & Menke 1976; Leclercq 1997) but resisted the temptation, arguing that the resulting genus would be huge and heterogeneous.
In a further complication, Australian species of Rhopalum break many of the rules that define the genus elsewhere. Keys used overseas don’t work here. Fortunately for me, Leclercq (1997) provides a list of features that, in combination, can be used to distinguish Rhopalum from Podagritus in Australia. And I’m also lucky that Paul studied French at school. Jean Leclercq, born 1921, is the seminal author when it comes to the taxonomy of Australian Rhopalum. And all their papers are written in French. Oh, and Google translate has also been quite helpful.
While only some of the features Leclercq lists are visible in my photos, I’m confident I have Rhopalum. The small size of the wasps is a clue, and wing venation is also convincing.
These are indeed small wasps – females around 7mm long, males closer to 4mm. Estimating size in the field is tricky. Here’s a technique I find works quite well.
Second question: which species of Rhopalum?
Rhopalum variitarse was obviously at the top of my short-list, simply due to their sand-nesting habits (Evans & Matthews 1971a; McCorquodale & Thomson 1989). Rhopalum don’t typically nest in the ground. Outside Australia they always nest inside plant material (Leclercq 1997) and the same is true for at least some Australian Rhopalum (e.g. R. bendorense nesting in pithy green stems) (Matthews 2000). There are a few other mentions of sand-nesting (e.g. R. frenchii & R. aliciae) (Turner 1915), but the behaviour of the vast majority of Australian species is completely unknown.
So our local wasp might be Rhopalum variitarse, but equally it could be one of around 100 other described Australian Rhopalum … or perhaps even an unnamed species. Pulawski (2010) reported seeing a dozen apparently undescribed species of Rhopalum in the Australian National Insect Collection (ANIC) – and even that collection may be incomplete.
Unfortunately our field photos don’t provide the detail needed for accurate species ID.
A DECISION POINT
and a little more fieldwork
At this point we faced a dilemma. Could we justify collecting (and killing) a wasp for species identification? And should we excavate her nest, emulating the study methods of Evans and others?
We decided to go ahead. Our photos and behavioural observations will be of most value to other researchers if the wasp species identity can be verified. In addition, a record of the prey species and the nest architecture will add significantly to what is known about Rhopalum nesting in Australia.
The dig in progress … 12th Sept, 2022.
So, with some reluctance, I captured a female and together Paul and I excavated her nest and unearthed her cache. Carefully. With a fine-haired paint brush. And forceps.
I’m sure we missed some details, and several of the buried flies were somewhat damaged in the process, but the results are nonetheless impressive. And apparently novel, at least in terms of the public record.
MICROSCOPY AND A LOT MORE READING
Specimens in hand, the next steps involved lab-based photography, microscopy, the application of taxonomic keys, and comparisons with species descriptions. While I have been wrestling with the taxonomy of Rhopalum, Paul has clocked up long hours studying the tiny flies we collected from her nest.
Here’s what we now know.
Rhopalum coriolum … and ours are perhaps the first photos ever published!
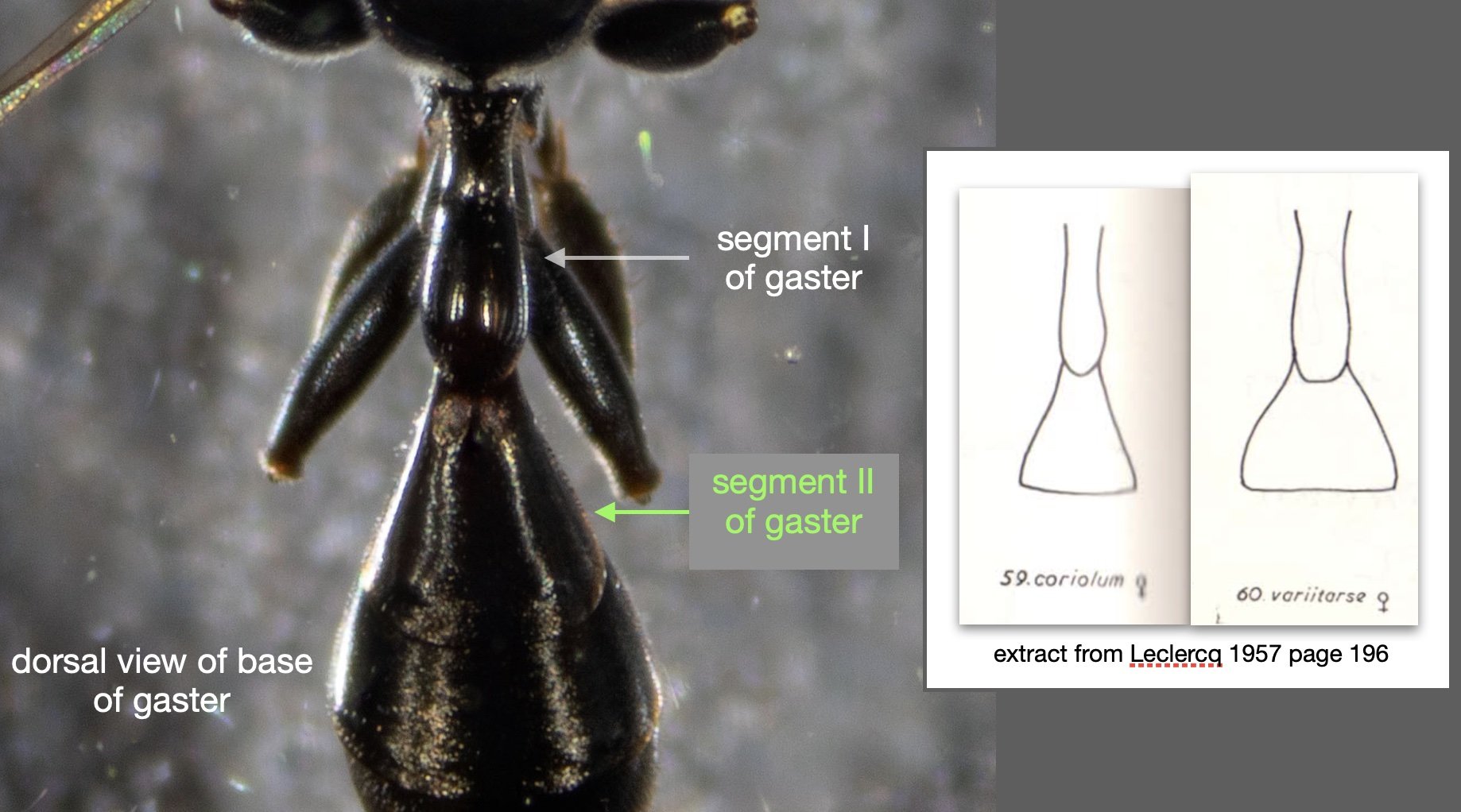
Success – and much excitement! Our wasp is a perfect match for Leclercq’s descriptions of Rhopalum coriolum (1957, 1997).
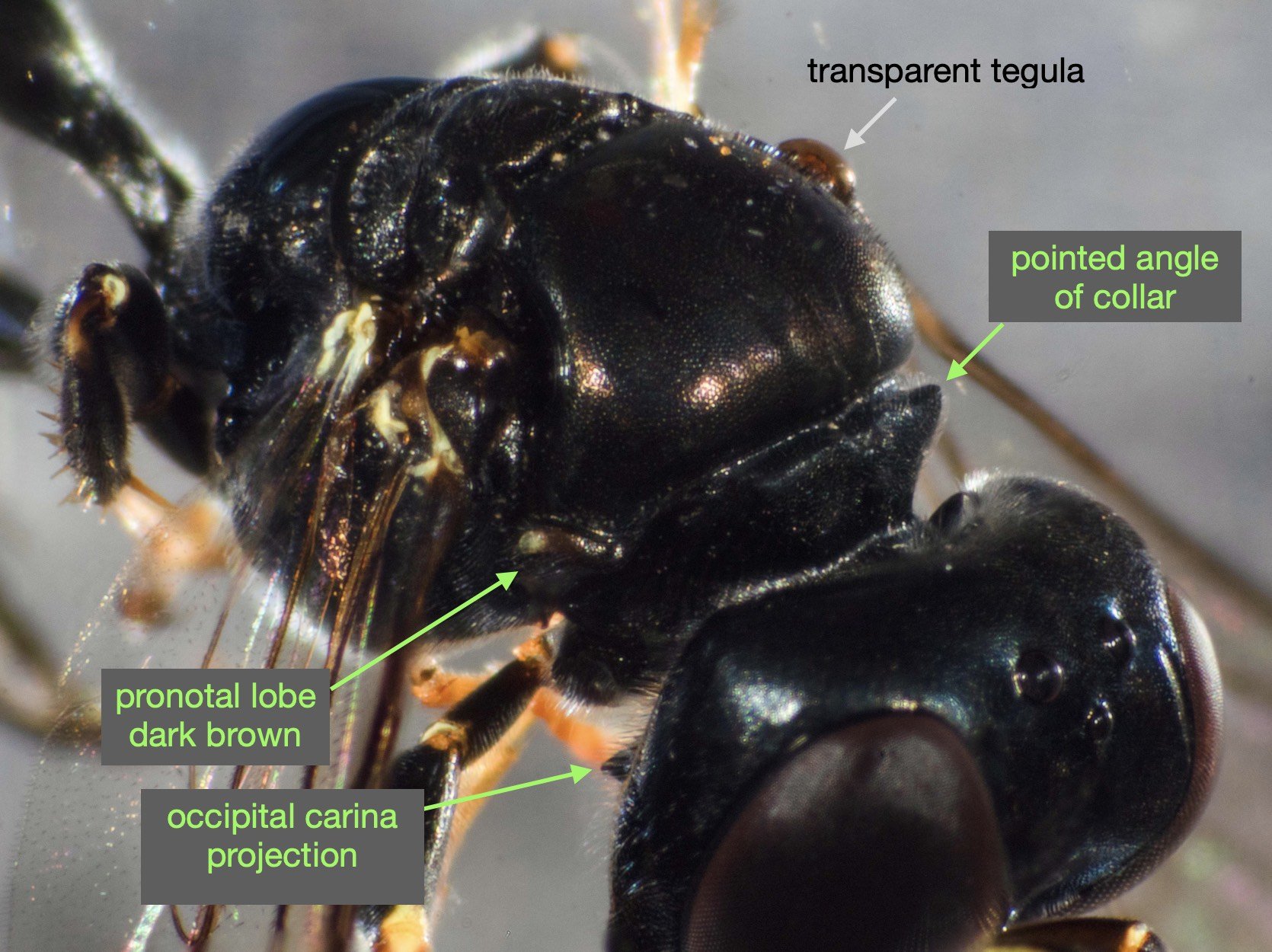
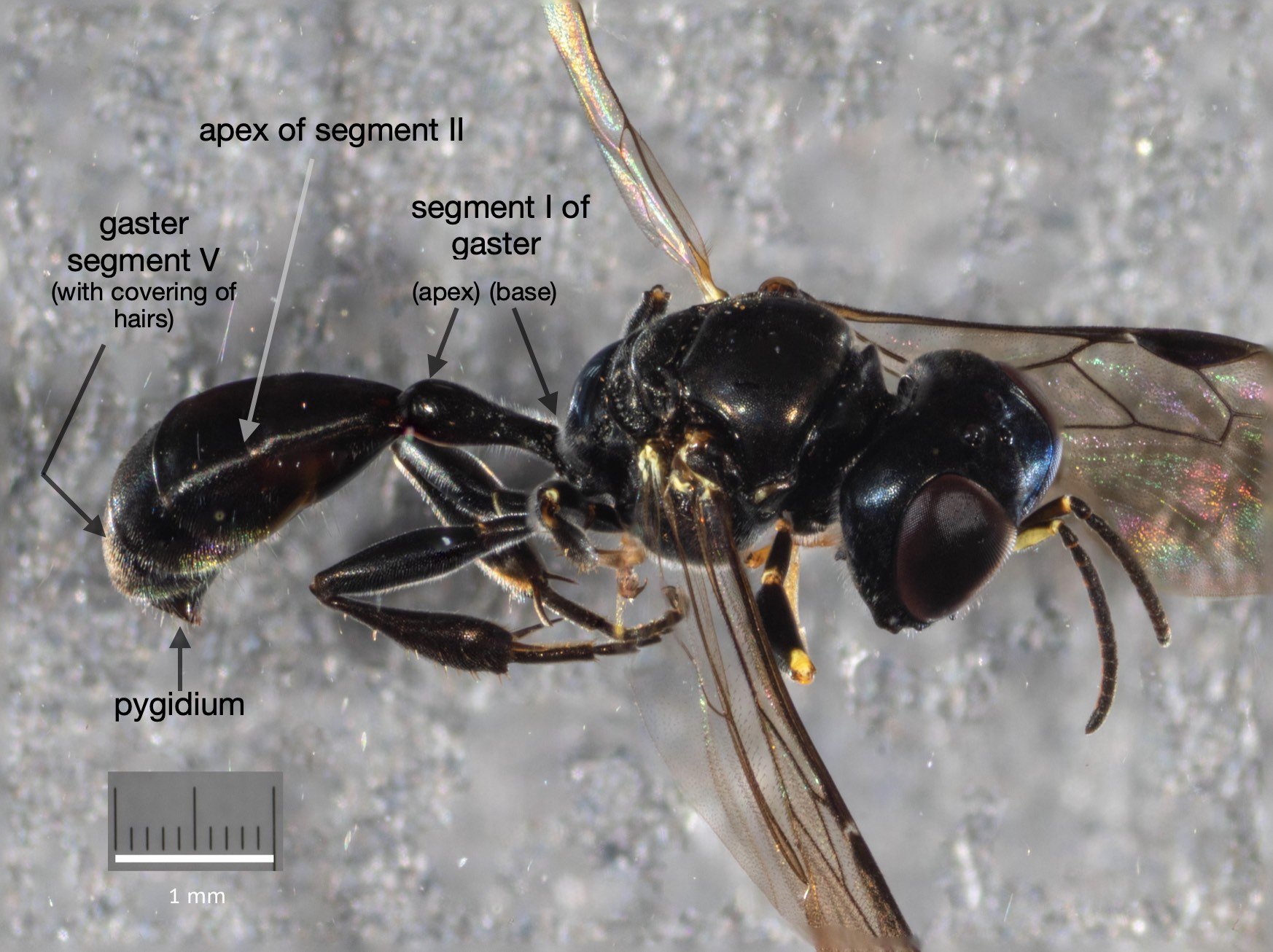
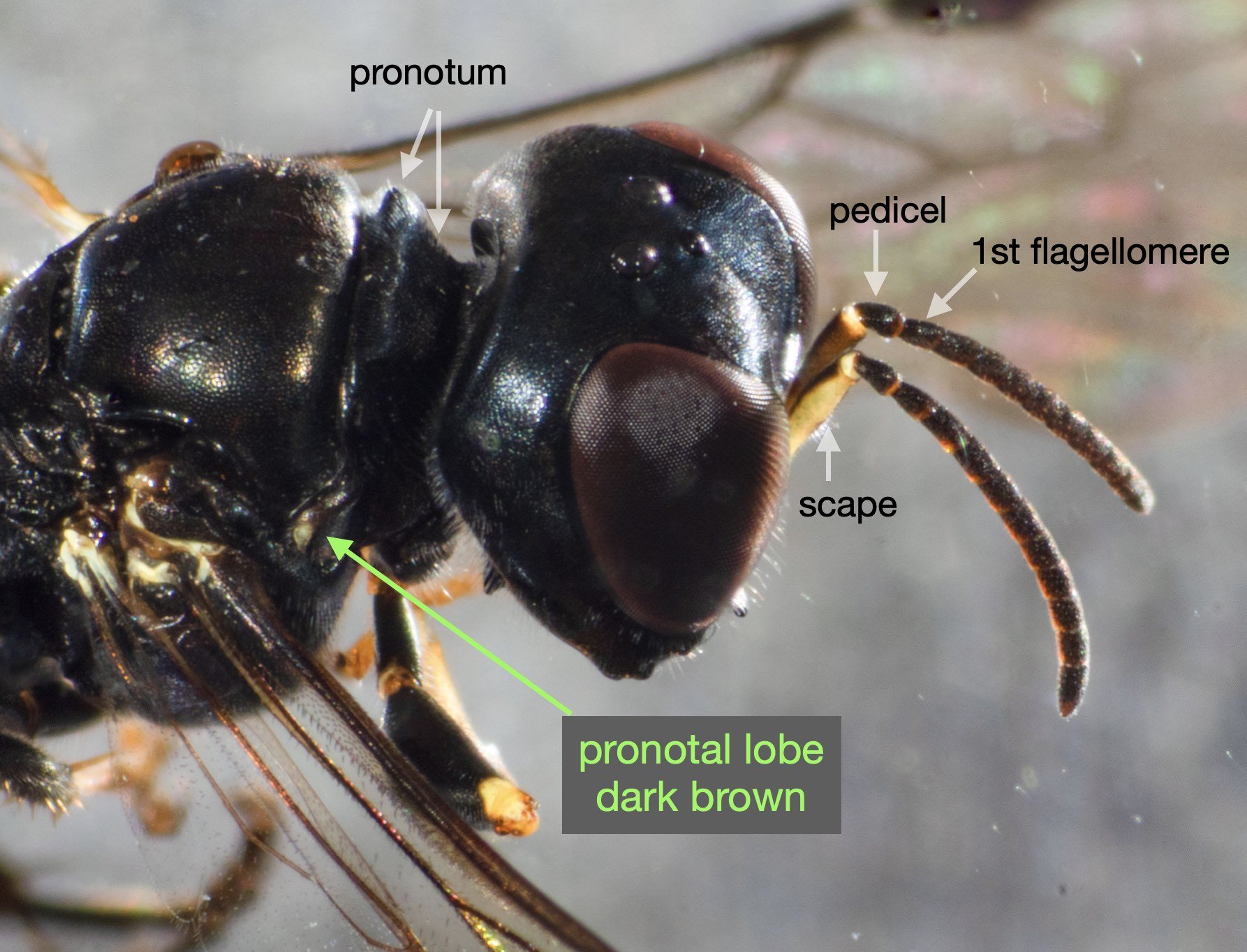
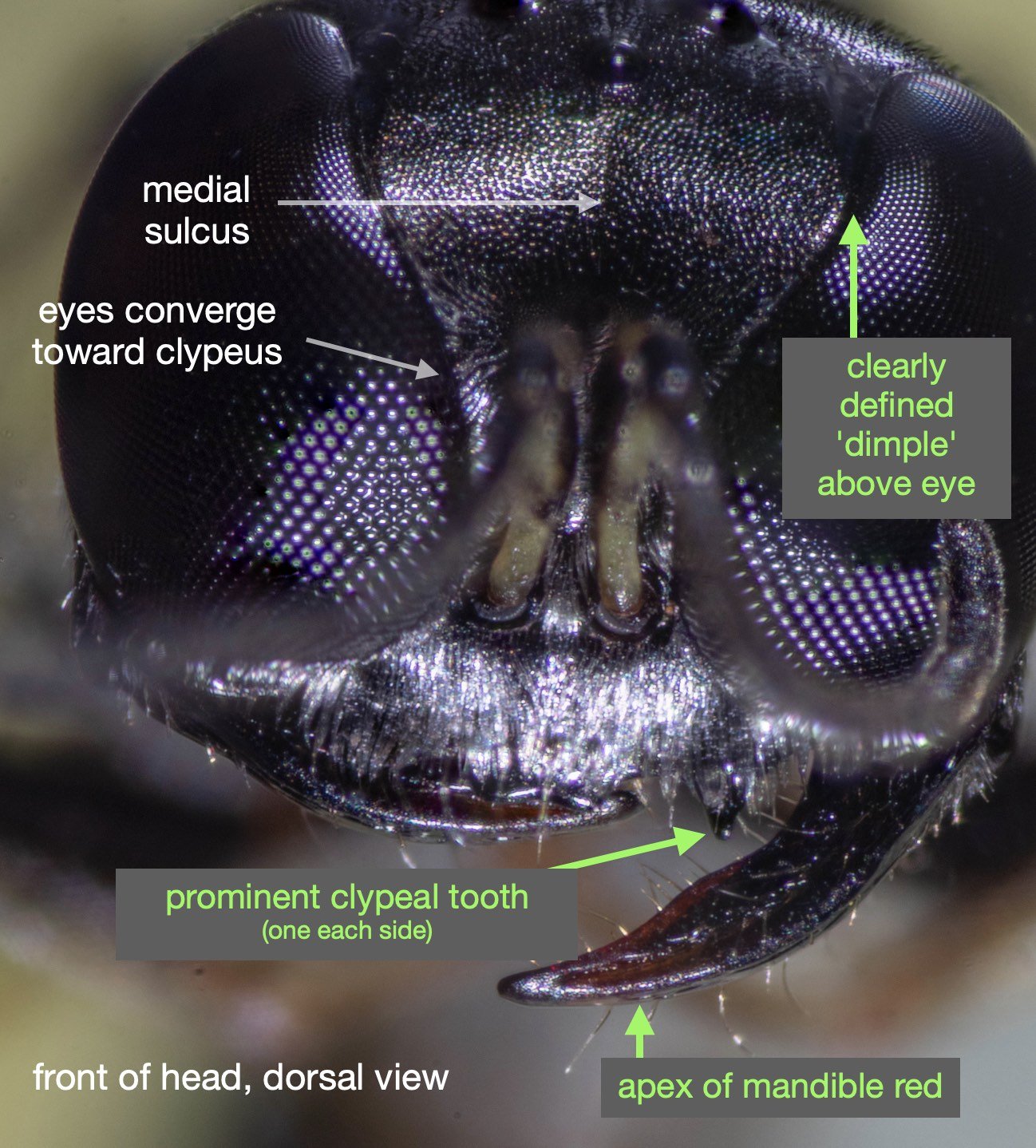
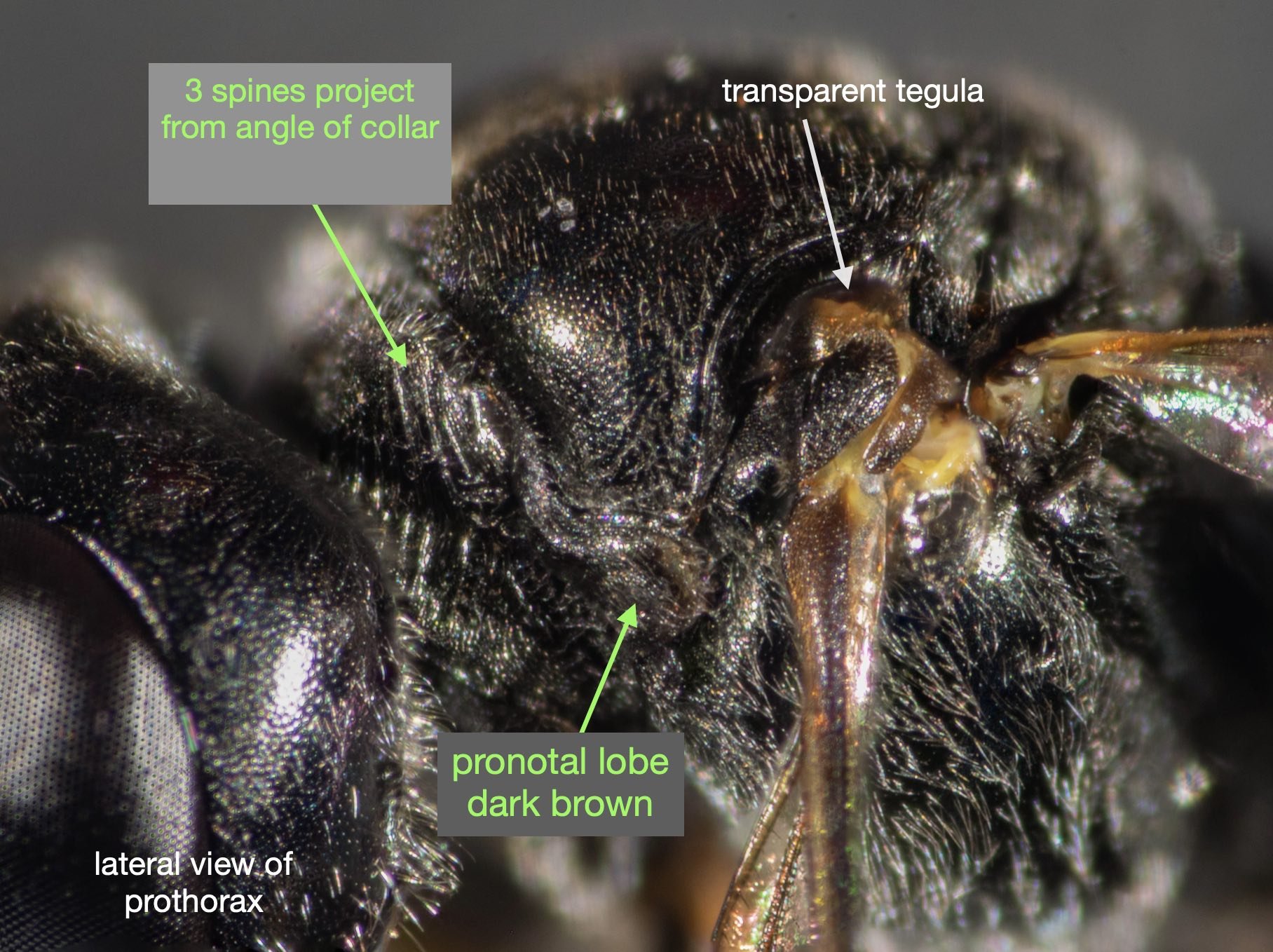
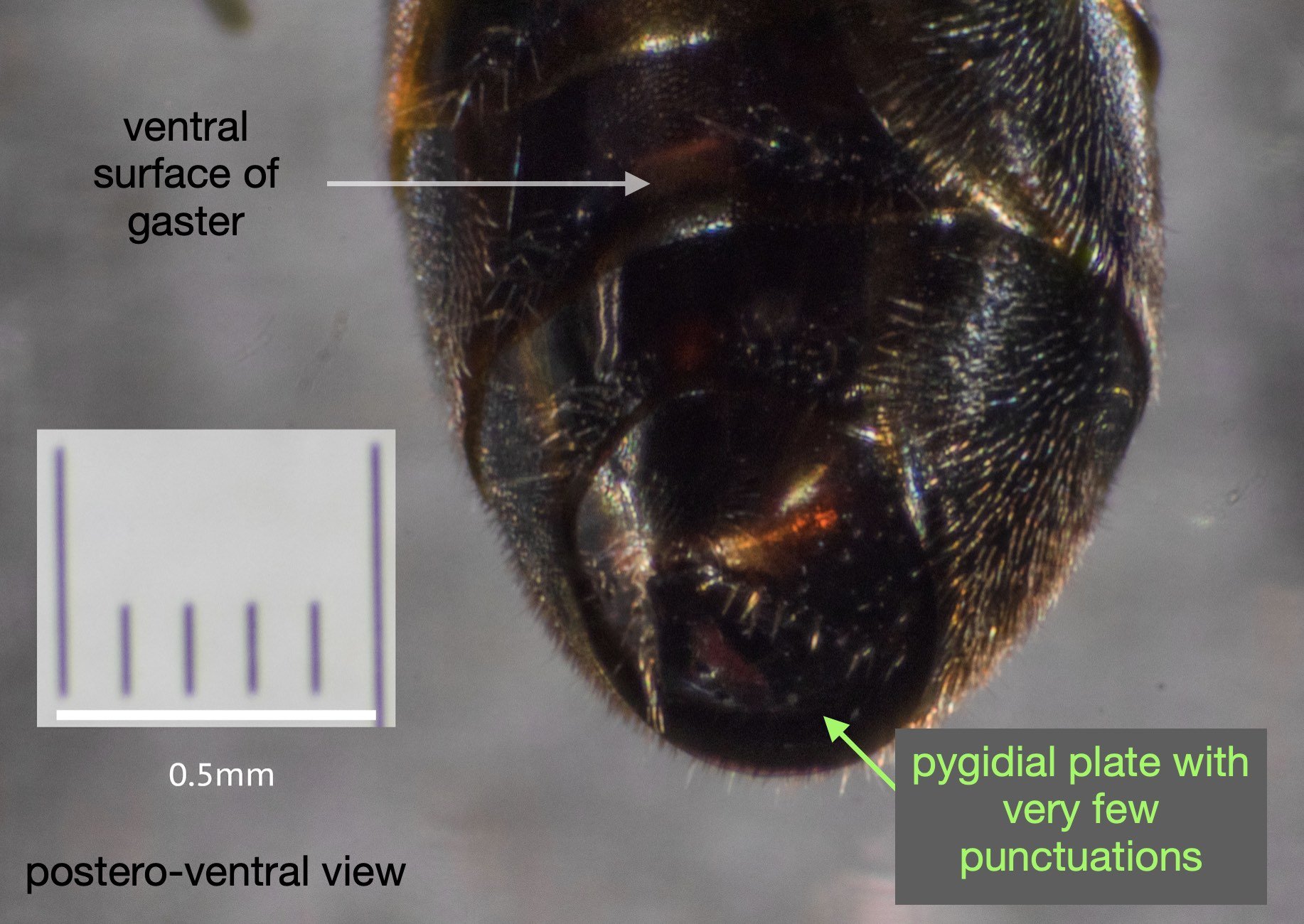
This species is very similar to Rhopalum variitarse yet the differences between the two species are distinctive … and readily apparent in our specimen (green text).
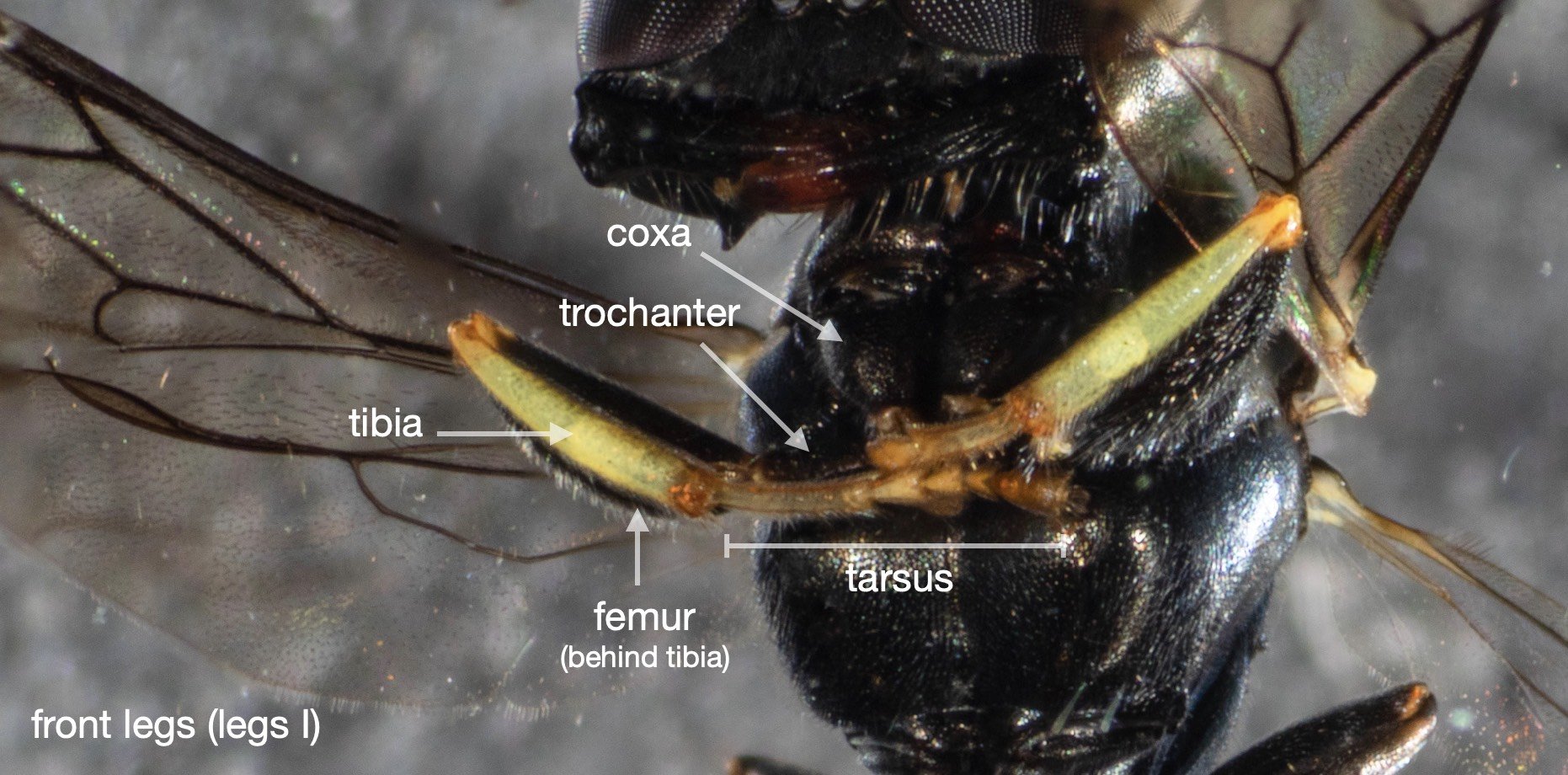
For the record (and for anyone else with a particular interest in such things) I’ve included more annotated images at the end of this blog.
Collecting a specimen has certainly proven worthwhile. We will soon deposit her with a museum … and I know of only one other specimen of Rhopalum coriolum held in public collections. That specimen is the holotype, the individual wasp upon which the original species description was made in 1957 (Leclercq 1957), collected decades earlier in the Blue Mountains near Sydney.
One caveat. I’m as confident as I can be of the identification. To be absolutely certain we would need to take a trip to Melbourne and compare our specimen with the holotype which is held by Melbourne Museum. Perhaps we will do just that. Or maybe we will find an interested researcher keen to take a look for us.
It seems that nothing is known about the biology of Rhopalum coriolum. Until now. Our observations of nesting behaviour and prey choice make an original contribution to knowledge of Australia’s crabonine wasps. A gratifying outcome indeed!
The nest and prey of Rhopalum coriolum
Our little female had constructed a three-room nest just a few centimetres below ground. We were surprised at the shallow position of the chambers. The Rhopalum variitarse nest excavated in Canberra by Evans and Matthews (1971a) was more than 10cm deep.
It seems likely that our local wasps were limited by the hard-packed clay layer at a depth of about 3cm.
As expected, each chamber was packed with paralysed flies, and in Cell 2 we discovered an egg. It is possible that one or both of the other cells also contained an egg, lost during our excavations.
The flies were definitely alive! Twitching mouthparts, flicking halteres and abdominal contractions – but no sign of leg movement. This did set Paul to wondering about the specificity of the wasp toxins, harking back to his research career in insect neurobiology. For another day, perhaps.
Also of particular note is the size of the egg and its location. It was close to 2mm long! That is rather large, considering the small size of the wasp. And she had attached it the throat (i.e. ventrally, between the head and thorax) of one of the flies … just as reported for Podagritus (Evans & Matthews 1971a).
The white egg discovered in Cell 2. It is attached to the larger of these flies, the dagger fly (Empididae).
[The small orange gnat (Cecidiomyidae) was also in Cell 2 and just happened to stick to the egg, probably as the cell collapsed during excavation]
Collection made on 12th Sept, 2022.
The diversity of flies is quite impressive. Seven families, ranging from tiny gnats to the larger, long-bodied dagger flies. Of course, none of them are very large!
Identification of flies is not trivial. Even with a specimen in the hand, getting them to family is often no mean feat. Paul sought a little assistance from the iNaturalist community and extends a special thanks to Tony D for his advice.
The mix of species does make me wonder about the wasp’s favoured hunting ground. The preponderance of Rivellia suggests she was targeting fresh marsupial droppings – we often see mating swarms of Rivellia gathering on wombat and wallaby poo. Or perhaps the wasp is simply attracted to species that swarm, more generally. Matthews made this suggestion regarding prey selection by Rhopalum bendorense (Matthews 2000).
A few other notes:
Some of her captured prey were far from defenceless. Empididae (dagger flies) are predators of various insects … including wasps!
Perhaps the dagger flies were themselves preying upon Rivellia, distracted, when the wasp found them.
One little fly may even have been nabbed while trying to poach the wasp’s prey. Milichiidae are often called ‘freeloader flies’, and many species in this family feed on the insects caught by others. Convoluted, I know, but ecological interactions are like that.
There is some overlap between the flies we found and the fly families already listed as known prey of Rhopalum. Both Lauxaniidae (Evans & Matthews 1971a) and Empididae (McCorquodale & Thomson 1989) have been collected from Rhopalum variitarse nests. However, given the wide range of families involved, it seems likely the wasps take whatever is available and of a suitable size.
Both of the crane flies were without legs, but all the other flies were intact. The act of detaching the very long legs of the crane flies was clearly a deliberate act on the part of the wasp. And she wasn’t the only one engaged in this behaviour. Several of my field shots show females of neighbouring nests carrying similarly legless crane flies.
That sneaky fly exposed: it is indeed a kleptoparasite!
Yes, I did eventually manage to catch one of the sneaky flies. It felt like quite an achievement – they are alert, and fast! It was given the ‘Paul treatment’, and we have a confirmed ID.
Apotropina (family Chloropidae), sometimes called ‘frit flies’. This family of flies is cosmopolitan and many species are known agricultural pests.
The identity of the ‘sneaky flies’ is revealed - Apotropina. The silvery colour of the living fly is lost when submerged in ethanol.
see iNaturalist record
I confess that at this point I was feeling a little disappointed. Most chloropids belong to two sub-families, both of which have predominantly phytophagous (feeding on plants) or saprophagous (feeding on decaying matter) larvae. Perhaps my kleptoparasitism hypothesis was wide of the mark.
A bit more reading and I’m encouraged again. Unlike most of its chloropid cousins, Apotropina belongs to “the enigmatic small subfamily Siphonellopsinae” (Marshall 2012 p. 370) – and these tend to be more meat-eating. But what really caught my attention was the statement that Apotropina is “a particularly common genus in Australia, where one species scavenges in the nests of sand wasps (Bembix)” (Marshall 2012 p. 371).
Aha! But what does scavenging involve? After a bit more Googling and paper chasing, I’m even more excited.
Apotropina is a known larval kleptoparasite of sand-nesting crabronid wasps!
And once again this knowledge comes from the careful observations published by Howard E. Evans and Robert Matthews.
Extract from Sivinkski et al., 1999, page 188
Extract from Evans & Matthews, 1971b, page 297
The genus known as Lasiopleura in Evans’ time was officially renamed Apotropina in 1980 (Sabrosky 1980).
So my hunch was right! Those sneaky little Apotropina females were indeed seeking to lay their eggs inside the Rhopalum coriolum nests. I can’t prove that they were successful, but they are certainly guilty of loitering with intent.
Thinking back, I remember seeing these flies before – they (and I) were hanging about Bembix and Cerceris nests at the time. Various sand wasps, including Bembix, should reappear in large numbers any day now. I’ll certainly be more attentive to Apotropina from now on.
References
Bohart, R.M. & Menke, A.S. 1976. Sphecid Wasps of the World: a Generic Revision. University of California Press, Berkeley.
Evans, H.E. 1966. The Comparative Ethology and Evolution of the Sand Wasps. Harvard University Press, Cambridge, Massachusetts (cited by Sivinksi et al. 1999)
Evans, H.E. & Matthews, R.W. 1971a. Notes on the prey and nests of some Australian Crabronini (Hymenoptera: Sphecidae). Journal of the Australian Entomological Society, 10: 1-4
Evans, H.E. & Matthews, R.W. 1971b. Nesting behaviour and larval stages of some Australian Nyssonine sand wasps (Hymenoptera: Sphecidae). Australian Journal of Zoology, 19: 293-310
Leclercq, J. 1957. Le genre Rhopalum (Kirby, 1829) en Australie. Bulletin et Annales de la Société Entomologique de Belgique, 93 (VII/VIII): 177-232 (available here)
Leclercq, J. 1997. Hyménoptères Sphécides Crabroniens d'Australie, du genre Rhopalum Stephens, 1829. Notes Fauniques de Gembloux, 32: 3-101 (available here)
Marshall, S.A. 2012. Flies: the Natural History and Diversity of Diptera. Firefly Books, Buffalo.
Matthews, R.W. 2000. Nesting biology of the Australian stem-nesting wasp Rhopalum benorense Leclercq (Hymenoptera: Crabronidae). Australian Entomologist, 27 (1); 1-4
McCorquodale, D.B. & Thomson, C.E. The prey of some Australian sphecid wasps (Hymenoptera). Australian Entomological Magazine, 16(4): 93-97
Pulawski, W. J. 2010. Rhopalum nasale, a new species from Australia (Hymenoptera: Crabronidae). Journal of Hymenoptera Research, 19(1): 139-143
Sabrosky, C.W. 1980. Unexpected synonymy in Chloropidae, from the family Ephydridae (Diptera). Australian Entomological Magazine, 6(6): 101-102
Sivinski, J., Marshall, S. & Petersson, E. 1999. Kleptoparasitism and phoresy in the Diptera. Florida Entomologist, 82(2): 179-197.
Turner, R.E. 1915. III. – Notes on fossorial Hymenoptera – XV, Annals and Magazine of Natural History, 15:85, 62-96 (link)
ADDITIONAL ANNOTATED IMAGES
All images show the same individual female, collected here on 12th September, 2022. She is at least 7mm in length. It is difficult to be more definitive due to the curvature of the body. Note that the size of the Rhopalum coriolum holotype (also a female) is 7.5mm (Leclercq 1957).