A bright, new skin

These past couple of months I’ve been studying life inside a lump of dried mud.
And it’s not nearly so dull as it sounds!
The massive nest belongs to a mud-dauber wasp (Sceliphron laetum), and many of the 19 nest cells did eventually yield the next generation of this large, distinctive species. But a parasitic invader had been at work too. Five cells had been taken over by cuckoo-wasps.
These little parasites (Chrysis lincea) are among the most eye-catching of wasps. Sometimes called emerald wasps, they have a brilliant, shimmering cuticle of blue and green.
Watching them develop, I’ve become quite fascinated by the process of cuticle development.
In both species, the formation of the cuticle normally takes place well out of sight – inside a multi-layered cocoon, deep inside the mud-nest. But by removing several larvae and pupae from their cocoons, I’ve been privy to their extraordinary metamorphosis.
As I watch on with wonder, the pale, soft-bodied insects are transformed to vibrant, thick-skinned adults.
So what am I actually seeing? How are the colours formed, and where?
In particular, what is responsible for the extraordinary iridescence of the cuckoo wasps (Chrysis)?
With a swag of photos in hand, I set out to learn more about the intricacies of insect cuticle development, and about Chrysis particular.
Cuticle structure and moulting
First, I needed a quick refresher on the structure of the insect cuticle.
In different parts of the insect body, and at different stages of its development, the layers of the cuticle vary in thickness, opacity, and elasticity. For example, there is little hardening of the procuticle during the larval stages, and even in the adult wasp there are thin, flexible areas with little or no sclerotisation. Without such areas, the insect would not be able to bend its appendages nor – in the case of adult Chrysis – curl into a defensive ball, protected by the hard, dorsal cuticular plates.
Insects periodically moult as they develop. It is necessary for growth and also at times of metamorphosis – changing from one body shape to another. In the developing Chrysis and Sceliphron, I was witnessing the final moult from pupa to adult.
cuckoo wasp (Chrysis lincea), cocoon removed (from Cell J, 30/1/22)
At the time I removed this Chrysis from its cocoon, it had already attained its basic adult shape – big eyes, long antennae, legs, distinct head, thorax and abdomen. But it’s not an adult yet. There is further body refinement – and another moult – to come.
The transparent outer wrapping is the cuticle of the pupa. It’s temporary but it serves an important function. It accommodates the developing adult body while protecting it from desiccation and pathogens. Proof? This vulnerable-looking pupa continue to develop successfully in a glass dish … no cocoon, no mud nest, no humidicrib.
After a bit more reading, I concluded that this immature pupa is already well into its final moult! Inside the thin layer of pupal cuticle, the final steps in the development of a winged adult are well underway. Internal changes I can only guess at … but the surface changes I can see. And it is this transformation of the cuticle that fascinates me.
Steps involved in insect moulting: a basic overview
Again, a few summary diagrams are useful at this point. Moulting involves much more than just shedding the old skin. There are steps that come before, and steps that come later, and the process is not entirely sequential. But basically, it goes like this:
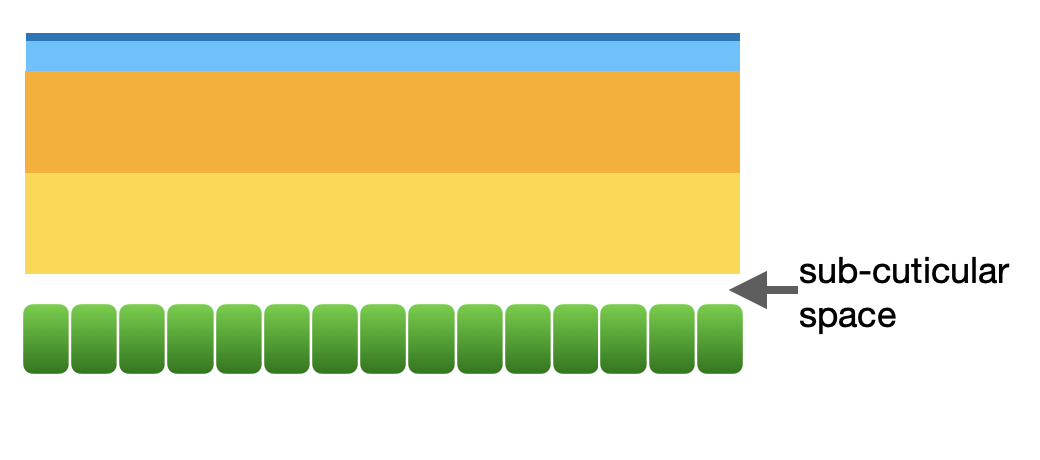
Step 1: apolysis
The epidermis is a continuous sheet of living cells. In response to hormonal signalling, the cellular machinery of these cells shifts into gear. The epidermal cells produce various enzymes and structural proteins and release these into the region beneath the endocuticle – thereby forming a visible ‘sub-cuticular space’.
Step 1 of moulting: apolysis
This sub-cuticular ‘space’ is therefore not empty at all. It is filled with transparent ‘moulting gel’. Some of the moulting enzymes digest the overlying procuticle … but not immediately. They are only triggered once the epidermal cells are protected by the formation of a new epicuticle (Step 2).
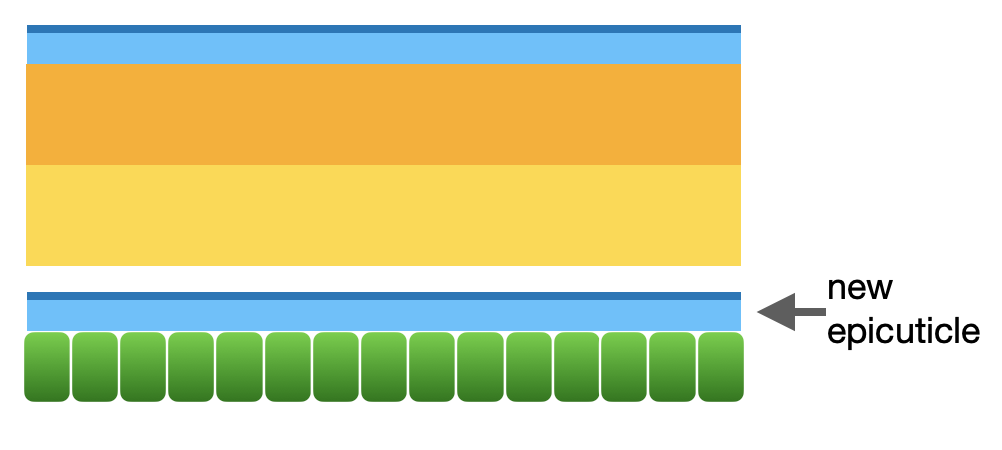
Step 2: new epicuticle forms
The first layer of the new cuticle to form (dark blue in diagram) will become the cuticulin layer. This is the layer which will ultimately determine the surface area and patterning of the new cuticle. In areas of the body that expand after moulting (such as the wings, in the case of the wasp’s final moult), this layer is wrinkled and folded. The inner epicuticle layer (pale blue) is then produced.
Step 2 of moulting: new epicuticle forms
Step 3: old endocuticle digested, new procuticle forms
With the protective new epicuticle in place, the enzymes in the sub-cuticular space are activated and they digest the old endocuticle. Around the same time, proteins and sugars for the new procuticle are deposited and assembled. Note that sclerotised regions of the old procuticle resist breakdown. The stabilised components cannot be readily recycled, and they are lost during the next step … ecdysis.
Step 3 of moulting: digestion of old procuticle & formation of new one
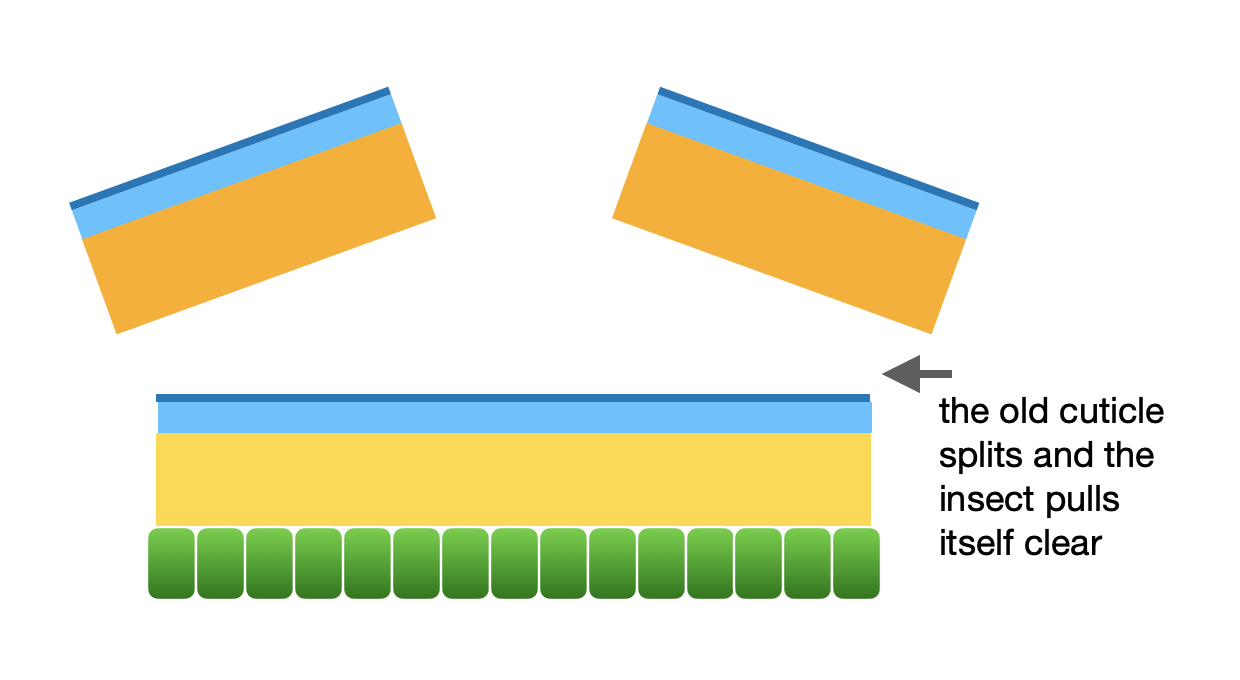
Step 4: ecdysis … the old cuticle is shed
The most obvious event in the moult is when the old cuticle splits and the soft-bodied insect emerges from the cast off skin. But this is not the end of the story.
Step 4 of moulting: ecdysis
The insect can now grow, as the new epicuticle is often highly folded and can also be stretched. For example, the wings. These are expanded after ecdysis, and prior to hardening of the cuticle.
Step 5: hardening, thickening & darkening
The procuticle is stabilised through molecular modifications: the cross-linking of proteins and/or the dehydration of the molecular matrix. This forms the exocuticle. Darkening is a side effect of this ‘sclerotization’.
Step 5 of moulting: development of the exocuticle
Note that not all areas of the cuticle become equally hardened or thickened, and it does not all happen at the same time. The process can take days.
So back to my little Chrysis. When I first popped the pupa from its cocoon, it had certainly reached Step 2 … and probably Step 3.
The final moult from pupa to adult was already well underway when I tore open the cocoon - although it would be weeks before the adult wasp would be ready to emerge.
Black arrows: blue spots and patches; White circle: sub-cuticular space particularly visible on this lateral process of the thorax
Chrysis lincea
The gap beneath the thin, outer skin (the pupal cuticle) is the sub-cuticular space – and it was already quite wide in places. Gleaming blue spots and patches are evidence of the new epicuticle (also see the close-up images, below).
The brilliant colours of the cuckoo wasp
With this basic cuticular structure in mind, I’ve been thinking about colour.
Chrysis went from creamy yellow to brilliant green in less than 3 days. And this was early in the moulting process (during Steps 2–3), a full week before ecdysis.
After losing myself (for weeks) in the literature on colour formation in insects, I’ve decided to be cautious in drawing any firm conclusions. The complexity of the chemistry and physics is daunting.. But there are a couple of statements that I am reasonably comfortable making.
The earliest colours to appear are the structural colours of the epicuticle
When I first uncovered the rather soft-bodied, yellow pupa it was already sparkling. This pale blue iridescence was clearly part of the new cuticle, not the overlying old one.
thoracic process in detail (30/1)
further detail (boxed section of previous image)
dotted line = pupal cuticle; solid line = new epicuticle
This metallic iridescence is due to the structure of the cuticle, not pigment. Such structural colours have been studied in a variety of insects, beetles and butterflies in particular, but rarely have they been documented for Hymenoptera.
Kroiss and colleagues (2009) analysed the cuticle of a European cuckoo wasp (Hedychryum rutilans) and showed that a multilayer reflector in the epicuticle was responsible for the metallic iridescence of the abdomen. And the reflector is indeed ultra-thin! It consists of a stack of six sheets, each just 185 nanometres thick. That is 0.000185mm! (Note that the visible light spectrum is 400-700 nanometres.)
Extract from Kroiss, Strohm, Vandenbem & Vigneron (2009), An epicuticular multilayer reflector generates the iridescent coloration in chrysidid wasps (Hymenoptera, Chrysididae). p984
A bit more background needed here: Colours are produced when light interacts with either pigments or microscopic structures.
Pigments are molecules that selectively absorb some wavelengths of light while reflecting others. The ‘colour’ we see is determined by the wavelength of light reflected. Pigments that reflect all wavelengths appear white, while those that absorb all wavelengths appear black.
In contrast, structural colour results from interference between the light waves reflected from different layers of the structure. Depending upon the angle of the light striking the surface, and the position of the viewer, the colour changes. This is, by definition, iridescence.
Sclerotization of the exocuticle begins very early in the cuckoo wasp’s final moult – and produces dark pigments
The thorax darkened rapidly, and this colour change soon spread down the dorsal surface of the abdomen. Such darkening is a clear sign of sclerotization of the exocuticle. Proteins are modified through a variety of chemical reactions. The result is a stronger, more stable cuticle. And pigments are formed. Brown colours through the sclerotization reactions, and black (melanin) through a concurrent yet distinct set of chemical reactions. The chemistry is complex, but for my purposes the point is this: in Chrysis, sclerotization begins at a very early stage of the final moult.
Chrysis lincea, with sclerotization of the new exocuticle well underway during Stage 3 of the final moult (11:20pm, 1/2/22 … just 30hrs after I popped it from the cocoon)
Yet Chrysis does not look black. It gleams blue and green, depending upon the viewing angle. Particularly once the wasp is free of the pupal cuticle.
Chrysis lincea, the same individual as in the preceding image, on its first day as a free-moving, active wasp. This would have been the day it broke free of the cocoon and mud nest … a full week after ecdysis (16/2/22)
The reason, of course, is that the darkly pigmented exocuticle lies beneath the epicuticle. So the black pigmented layer serves as a wonderful backdrop to the overlying structural iridescence … absorbing any stray light that passes through the epicuticle, leaving just the pure structural reflectance.
The sculpturing of the cuticle, with many circular pits across the dorsal plates, adds yet another dimension to the finished product. Chrysis is a truly beautiful little insect.
A local relative
The mud nest filled with Chrysis and Sceliphron wasps was not collected locally. We found it in northern NSW and simply brought it home to study. Neither are species we know from home, although both are common and widespread across Australia. However there is a local cuckoo wasp we often see here in the forest – Primeuchroeus.
Primeuchroeus, collected here in the forest just one month after the January 2020 fire.
I think it’s safe to assume that cuticle development and colour production take place the same way in both Chrysis and Primeuchroeus. The two species are very similar in appearance and are closely related.
Primeuchroeus - see iNaturalist record of Nov, 2019
I’ve yet to bear witness to the development of Primeuchroeus. While little is known about their biology, I think our local species seeks host nests inside logs or under bark. That is where we most commonly see them, poking about on standing or fallen trees. Perhaps I will investigate further. And perhaps one day I’ll accidentally raise one from a nest … just as we raised the glorious mutilid wasps last year (see Dec 2021 blog A window into mud-nests).
Key references
Andersen, S.O. 2010. Insect cuticular sclerotization: A review. Insect Biochemistry and Molecular Biology. 40: 166-178
Evans, H.E. 1984. Insect Biology: A Textbook of Entomology. Addison-Wesley Publishing, Massachusetts.
Hopkins, T.L. & Kramer, K.J. 1992. Insect cuticle sclerotization. Annual Review of Entomology, 37: 273-302
Kroiss, J., Strohm, E., Vandenbem, C. & Vigneron, J-P. 2009. An epicuticular multilayer reflector generates the iridescent coloration in chrysidid wasps (Hymenoptera, Chrysididae). Naturwissenschaften, 96: 983-986