Perginae larva hub

Workbook
This page serves as a hub, providing background and links to a series of pages dealing with the identification, life history and rearing of larvae in the subfamily Perginae.
In his ground breaking revision of adult sawflies in the genus Perga, Benson noted that “the larvae of very few species have been described at all, despite the very great interest that surrounds their colonial habits” (Benson, 1939).
Our knowledge of pergine larvae has progressed little in the intervening 86 years. There are very few published descriptions of the 57 species in the 8 genera of this subfamily.
Descriptions of insect larvae in general pose considerable challenges. As larvae typically have a simple body form, there are relatively few morphological characters available for taxonomic diagnoses. In addition, a larva often undergoes substantial changes in colour as it progresses through its various instars.
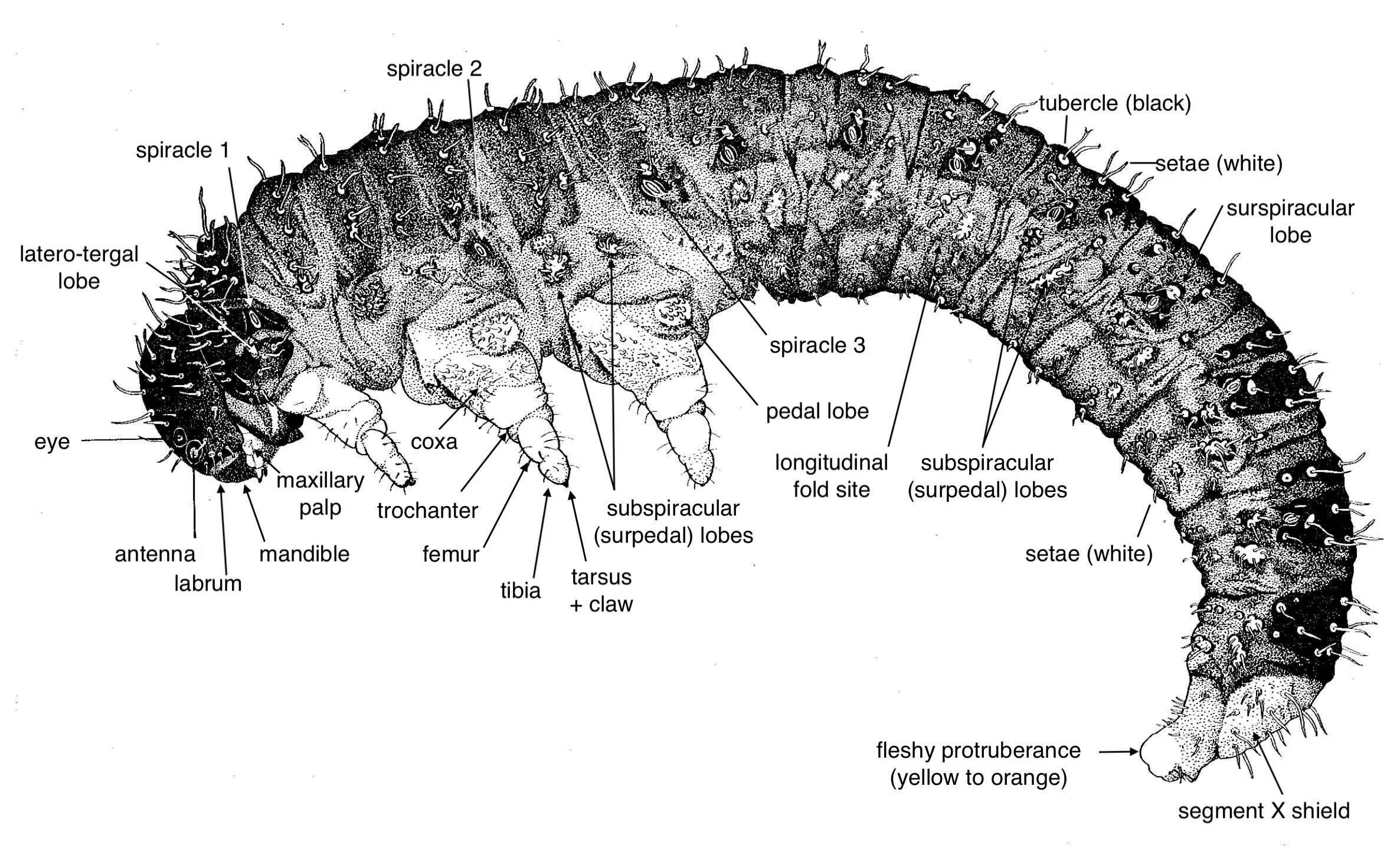
On this hub page, I describe the morphology of a generalised pergine larva - drawing on the few published descriptions.
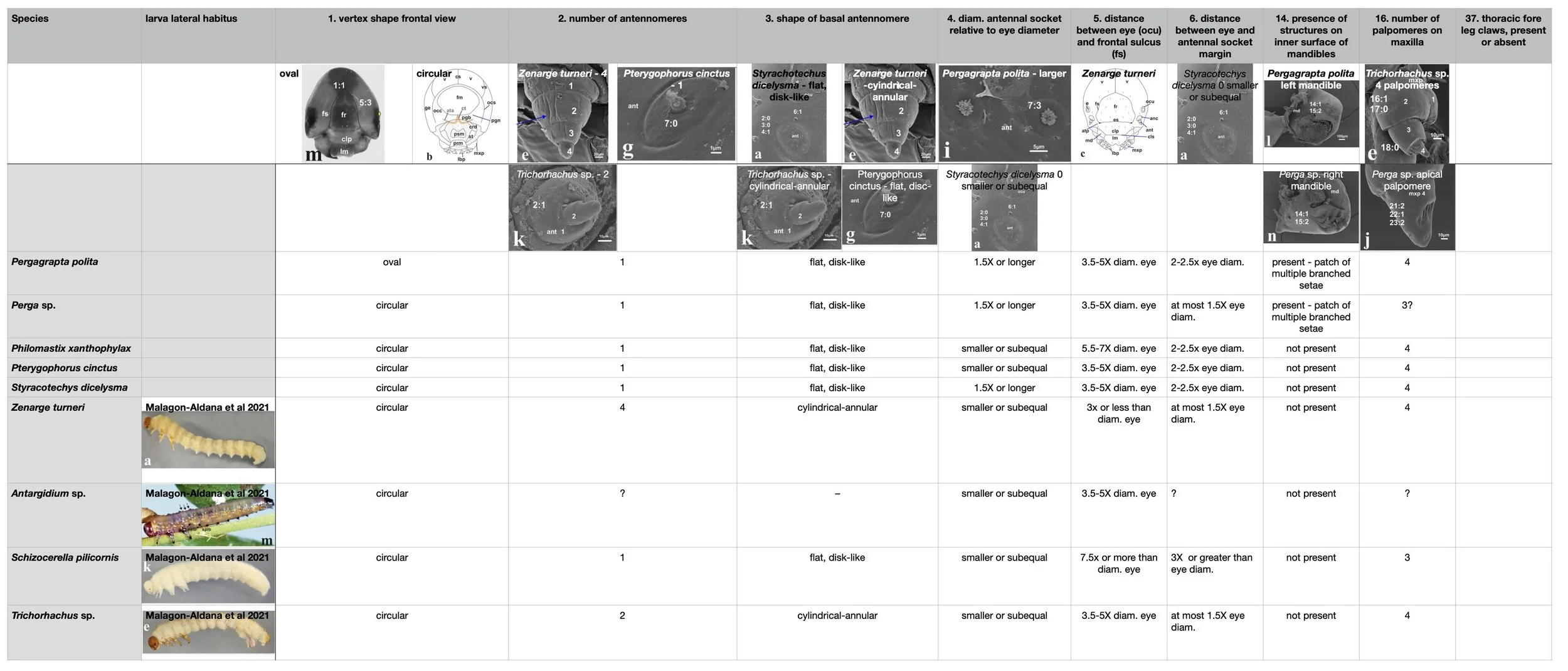
Comparison of morphology of Perginae larvae - from Malagon-Aldana et al. (2021)
add matrix showing structural features that differ between Perga sp., Pergagrapta polita, Philomastix xanthophylax and Pterygophorus cinctus
Click on image below to download a 2.9MB pdf.
Perginae larval rearing attempts
Perginae larvae batch 1
- collected from Princes Highway near Wingan River turn-off - iNaturalist observation and later iNaturalist observation
Perginae larvae batch 2
- collected from Old Bridge Road, Sept 12, 2025. This clutch was housed in a metal cage and fed eucalyptus leaves collected from saplings near the collection site - mainly E. sieberi. These were replaced every few days and the stems pushed into a florist block in the centre of the cage. This block was watered regularly to keep the vegetation fresh. A deep layer of dead eucalypt leaves was placed in the bottom of the cage surrounding the florist block, contained within a plastic lining.
The larvae showed a regular pattern of activity, resting in a cluster on a leaf during the day. Starting around 5pm they would begin eating the leaf and when that was consumed, they would disperse towards new leaves. By early morning they had moved back into a rosette cluster on a single leaf.
This pattern of behaviour continued until 2nd October, 20 days after collection, when the clutch split into two clusters, both low down on the cage on the wire below the level of the plastic lining. One cluster subsequently moved up onto the vegetation but by the morning of 3rd October, all of the larvae had disappeared. Slight scratching sounds were heard between the wire and cage liner until 6th October.
The cage was emptied on 9th October and all of the larvae were found dead in the bottom of the cage beneath the plastic liner.
Perginae larvae batch 3
- collected from Eucalyptus consideniana on edge of Kizzy’s block on Sept 14, 2025. We housed this clutch like this one in a metal cage and fed them eucalyptus leaves collected from the same tree on which we found them - a Yertchuck (E. consideniana). These were replaced every few days and the stems pushed into a florist block in the centre of the cage. This block was watered regularly to keep the vegetation fresh. A deep layer of dead eucalypt leaves was placed in the bottom of the cage surrounding the florist block, contained within a plastic lining.
We added two separate clutches of larvae from this same tree. The first batch of ~15 larvae (shown in the image above) was collected during the day on 14 September. The side branch on which they were clustered, about 1.5m from the ground, was cut and placed in the florist block, together with additional branches from the same tree. These larvae showed the same regular pattern of activity as the 313502193 larvae, resting in a cluster during the day on the base of a stem and moving as individuals to leaves at night to feed. Then 6 days later, on 20 September all of these larvae had headed into the leaves at the bottom of the cage.
On that same day, at 10:30pm, we collected the remaining larvae from the same tree as the first clutch. These were dispersed in small groups among the young foliage at the top of the sapling, about 4-5m above ground. We added them to the same cage. These showed the same pattern of grouping into a single mass during the day (although there were numerous outliers in the leaf litter) and dispersing to feed at night. 3 days later, on 23 September the activity of the cluster appeared disrupted. Only a fraction of the larvae grouped together in a cluster on vegetation, while others remained hanging on wire or amongst the leaf litter. A week later, on 30th September only a small group of 6 larvae was seen feeding on the foliage during the day. This small cluster continued to feed at night and regroup in a resting group during the day but on 7th October they too had disappeared. On 9th October we emptied the cage and found all of the larvae dead, scattered amongst the leaf litter. There was no sign of pupae.
Perginae larvae batch 4 - first successful rearing attempt
collected from E. globoidea sapling at start of Lot 5 track on Oct. 9th, 2025
iNaturalist observation of larvae, iNaturalist observation of beginning of pupation
first adult, a female #PW100, emerged on Feb. 17th, 2026. iNaturalist observation. Pupal duration = 107 days. Sawsheath hair morphology indicates ID of Perga dorsalis.
Perginae larvae batch 5
collected from E. globoidea sapling close to batch 4 sapling on Dec. 6th, 2025
Tips on rearing larvae in cages
Effect of number of individuals in colony - Carne (1969)
Carne (1969) p.119 “When larvae were placed on uncaged foliage in the laboratory, it was unusual for any members of a colony to survive to maturity unless the colony consisted of more than 20-25 individuals. Larvae dropped or wandered from the leaves and were, seemingly, unable to relocate the shoot. Larger colonies remained on the foliage, and few larvae became isolated. Similar results were obtained in studies on the effect of colony size on sawfly development (Dr. R. D. Hughes and Miss Josephine M. Walker, personal communication). These workers reared sawfly larvae either singly or in groups of 5-10. Although the cages used did not allow the larvae to move away from their food supply, isolated larvae gained less weight, took longer to mature, and survived less well than those reared in groups”.
Communication between larvae - Carne (1962)
p. 22 “Physical contact between individuals is maintained at all times during larval life. During movement each larva constantly checks on the presence of a follower by tapping with its hardened terminal abdominal sclerite (Fig. 9). If an individual strays from the moving column and fails to make contact with another larva, it manifests disturbance by an abrupt increase in its rate of tapping. The larvae in the main body of the colony respond immediately by uncoordinated tapping for a period of 10-15 sec. There is usually an "answering" signal from the stray, then further tapping on the part of the colony. It seems certain that this is a form of communication for it invariably results in the individual rejoining its colony. This it does by moving short distances and then pausing to tap and receive a response before moving on. Contact with the colony is achieved in a very short time, and with great economy of movement. As larvae do not react to quite loud noises, but are very sensitive to any unnatural movements of the branch they rest on, it is postulated that they respond to the low frequency vibration rather than to the sound produced by the tapping. Such a postulate is consistent with the rapidity and certainty with which the individual locates its colony. Acoustic orientation would be useless where straying occurs among the multiple dichotomies of foliage. Once the stray has rejoined its colony, nervous activation subsides abruptly and the colony becomes almost motionless, except for spasmodic twitches and for occasional gentle tapping by its peripheral members.”
p.23 “If one considers the extreme gregariousness of larvae, and the fact that the movement of most individuals is initiated by stimuli received from others, it would appear that the larvae which lead colonies to and from their resting sites play a special role. Certain individuals in colonies held under laboratory observation were found with unexpected frequency to be among those leading the colony.”
Feeding activity - Carne (1962)
p. 27. “Feeding began in the late afternoon, reached a peak at midnight or shortly after, and declined abruptly after dawn. Little or no feeding occurred during the hours of daylight. As larvae moult to later instars, they begin feeding later in the day and feed for a shorter total time.”
Mortality during inter-tree dispersal - Carne (1969)
p. 120 “The resulting mortality was greatly influenced by the prevailing weather, and by the time spent by the colonies on the ground. The latter was, in turn, influenced by the density of the tree stand, and by the nature of the ground surface and herbaceous cover. Most dispersals began during the night or early morning. Where trees were closely spaced (e.g. 10-30 ft apart), and the ground surface was smooth, the colonies frequently established themselves on another tree within a few hours, and suffered little mortality in the process. However, where the trees were more sparsely distributed (e.g. more than 50 ft apart), or where irregularities of the ground surface impeded their movement, the colonies were often still moving across unshaded ground during the hottest time of day. The larvae became weakened, and usually died of desiccation. Few colonies successfully moved distances exceeding 10-15 ft except on bare ground or on hard-grazed, level pastures.”
Mortality during soil entry - Carne (1969)
p.121-122 “The larvae encounter a period of considerable stress when they attempt soil entry. To survive, they must enter the soil quickly and begin construction of the cocoon which protects them from continued water loss. However, they are inefficient burrowers and penetrate into hard soil only with the greatest difficulty. Drought during the late winter and early spring, at which time the soil-entry stage occurred, resulted in high mortality of the larvae by causing the soil surface to set as a hard crust. The spring of 1963 was a period of drought and, during a survey of the study region made in October of that year, tangled masses of desiccated larvae were seen on the ground below most of the trees which had been heavily infested. Soil type undoubtedly influenced the distribution of P. a. affinis: it was never found in high numbers in areas where heavy clay soils predominated. The size of the colony greatly influenced its success in entering the soil. Most or all of the larvae in small groups died before doing so. However, in large groups, any point of entry was quickly exploited. Examination of samples of cocoon masses formed in dry sites showed that desiccation mortality was most severe at the periphery of the mass, suggesting that the bodies of those larvae which assisted in the breaking of the soil crust, but which did not themselves survive, contributed to the survival of those in the centre of the mass. The larvae achieved soil entry with the least mortality when the soil was soft, and the surface layer easily penetrated. Under small woodland trees there was rarely any litter, and the surface was usually packed hard by the treading of stock. The deep litter commonly present under old woodland trees prevented the caking of the mineral soil surface, and even under dry conditions the larvae were able to penetrate with comparative ease. Mature trees often provided cocooning sites for colonies which moved to them from younger trees in the vicinity.”
Host plant information for Perginae
following table from Schmidt et al. (2006)
References:
Carne, P.B. (1969) On the population dynamics of the eucalypt-defoliating sawfly Perga affinis affinis Kirby (Hymenoptera). Australian Journal of Zoology 17: 113-141.
Malagon-Aldana, L.A., Shinohara, A., Smith, D.R. et al. (2021) Comparative anatomy of the larvae of argid sawflies (Hymenoptera: Argidae): a phylogenetic approach. Org. Divers. Evol. 21, 361–392.
Meyer-Rochow, V.B. (1972) Verständigungsweisen bei koloniebildenden Blattwespen- und Käfer-Larven. Zeitschrift für Tierpsychologie 30: 451-455.
Meyer-Rochow, V.B. (1974) Structure and function of the larval eye of the sawfly, Perga (Hymenoptera). Journal of Insect Physiology 20: 1565-1591.
Schmidt, S., Walter, G.H., Grigg, J. & Moore, C.J. (2006). Sexual Communication and Host Plant Associations of Australian Pergid Sawflies (Hymenoptera, Symphyta, Pergidae). In Blank, S., Schmidt, S. & Taeger, A. (eds), Recent Sawfly Research: Synthesis and Prospects. Goecke & Evers, Keltern, pp.173-193.
This is a workbook page … a part of our website where we record the observations and references used in making species identifications. The notes will not necessarily be complete. They are a record for our own use, but we are happy to share this information with others.