Getting to know funnel ants

A swarming flight of thousands of ants last spring set me on a journey I’ve long postponed – to learn more about the role of ants in the forest.
I’ve taken ants for granted, perhaps because they seem ever-present and numerous. It’s the showy butterflies, ephemeral wasps, and intriguing bees that usually catch my attention. Large, fearsome-looking bull ants I’m always keen to photograph … but what about all the little ones?
An October ant swarm prompted me to make a start. Hundreds of unfamiliar, fat-bodied ants in flight – and I knew nothing about them! So I took plenty of photos and collected a few specimens.
Over recent months, I’ve been piecing together the story.
Who are they? What is their place in the forest’s ecology?
And what was behind the mass swarming of winged ants we observed in October?
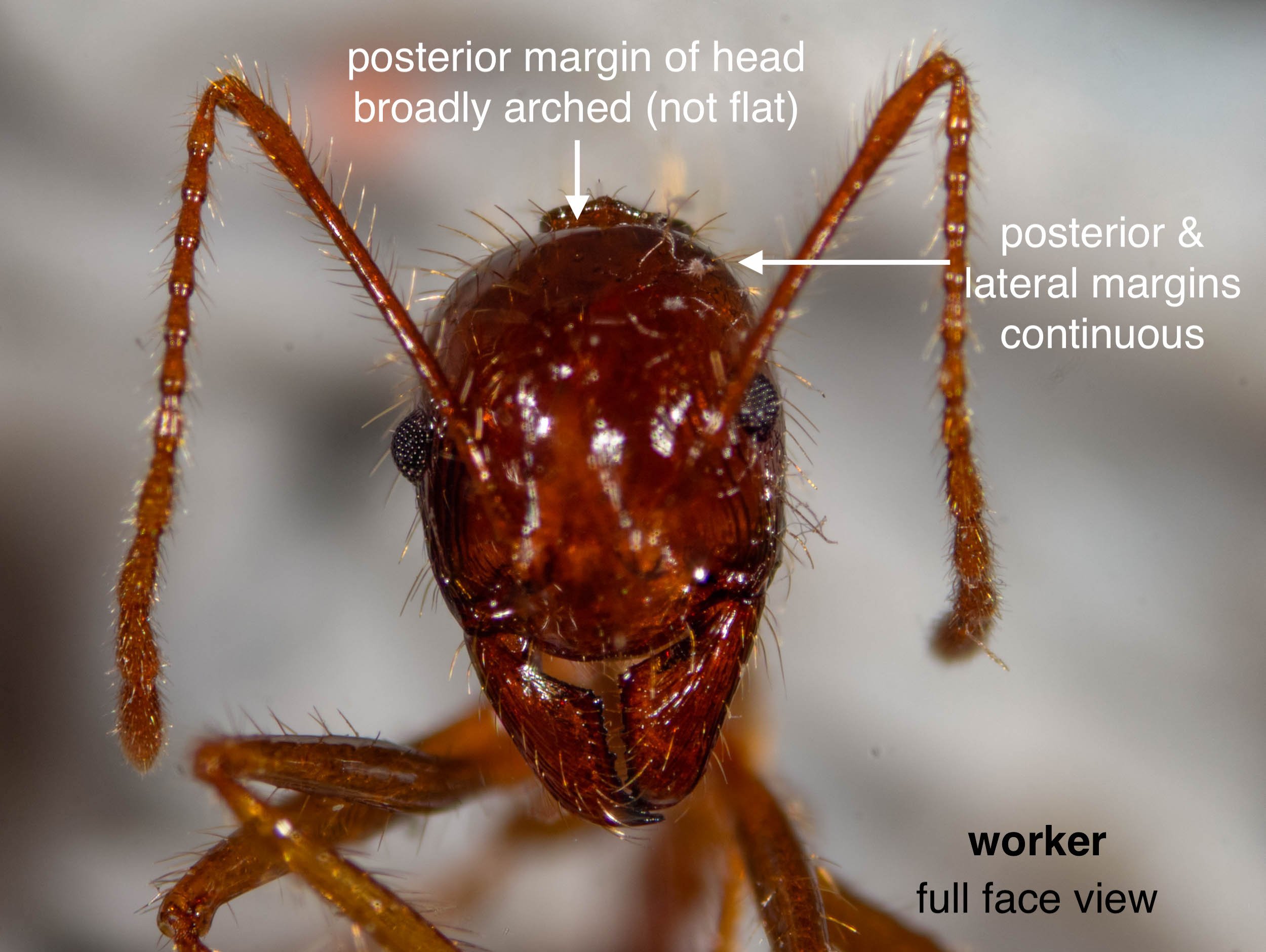
First, their identity
Funnel ants – a rather infamous native species
Based on the workers I collected, the ants key out as Aphaenogaster longiceps. It is a widespread and well-known Australian endemic. Notorious, in fact.
In certain situations these ‘funnel ants’ are considered a pest, undermining lawns and golf courses. Although Aphaenogaster longiceps is a native species, pest control companies are keen to help householders eradicate them. Enter ‘funnel ants Australia’ in a search engine and you’ll see what I mean!
Worker Aphaenogaster longiceps … post mortem.
Secretive ants with prominent nests
Aphaenogaster longiceps are widespread, common, medium-sized ants yet they are easily overlooked. They spend most of their lives hidden away underground. Individuals sometimes emerge at night to forage near the nest, but during the day I’m unlikely to see them … except in special situations. More about that later.
Funnel ants (Aphaenogaster species) are generalists – they eat sugars, dead insects, fatty seeds – but they’re unlikely to invade a picnic table. Much of their feeding is done inside the burrow, either the secretions of root-feeding aphids or the remains of wandering invertebrates that become trapped by the nest funnel and are dragged underground. Solitary foragers venture out in search of dead insects and fallen seeds, but they don’t gather above ground in large numbers (Shattuck 2008).
Aphaenogaster longiceps live in a wide variety of habitats across south-eastern Australia, including dry sclerophyll forests like ours. And although the ants themselves are rather secretive, their mounds are a common sight. In sandy soil, the nest entrances form wide, deep funnels (hence their common name). Under some conditions, dense aggregations of colonies riddle the ground with subterranean burrows (hence their status as pests of suburban lawns) (Shattuck 2008).
Funnel-shaped mound surrounding opening to Aphaenogaster longiceps nest (22/10/2021 11:23)
Two adjacent nest entrances, almost certainly belonging to the same colony. The sandy soil of our home forest is well-suited to this species. They tend to avoid rocky or wet areas. (6/11/2021 10:56)
Second, their ecological story
Pest-control interests aside, the ecological research literature on funnel ants is extensive. Topics range from the ants’ role in soil mixing (bioturbation) and post-fire erosion, to the collection and redistribution of seeds from wattles and other ‘myrmecochorous’ plants.
The take-home message: these tiny ants make a significant contribution to the structure and health of forest ecosystems.
Forest gardeners
Our local funnel ants are certainly not pests. In fact, I’m starting to think of them as gardeners. Let me explain.
Hundreds of Australian plants produce seeds with ant-attracting structures (elaiosomes). These form in various ways, and have evolved independently in different plant families. Typically, they are lipid and protein-rich structures that play no direct role in seed germination. They do, however, play an important role in seed dispersal.
Mature, split fruit of Acacia terminalis. Note that the seeds of some pods have already been shed, but are caught in adjacent spider webs (arrows).
(December, 2011)
Each ‘diaspore’ of Acacia terminalis comprises a smooth, black seed and a pale yellow elaiosome (arrow)
(same tree as in adjacent image)
Foraging ants carry these parcels back to the nest to feed their larvae. The seed itself usually has a strong covering and so remains undamaged, its contents unattainable. Once the elaiosome is removed, the ants discard the seed. The plants benefit from this partnership. Their seeds are more widely dispersed than they might otherwise be. They are also quickly protected from predation and from future fire. Even a thin layer of soil can be enough.
Seed dispersal by ants (myrmecochory) has been extensively studied, and from a variety of perspectives. What type of plants and what ant species are involved? How does it benefit the plants? How do ants find and choose seeds, and what do they do with them when they’re finished? How far do they carry them? Do some plants cheat, fooling ants without rewarding them? How does myrmecochory affect seed bank stores and plant survival in fire-prone environments?
After a brief dive into the literature, I’m now confident that funnel ants play a role in forest plantings here at home. The lines of evidence are these:
Funnel ants are known seed collectors.
Aphaenogaster longiceps is recognised as an active collector of elaisome-bearing seeds (Anderson 1988; Hughes & Westoby 1992; Hughes et al. 1994), including Acacia species (Berg 1975).
After removing the elaiosomes, Aphaenogaster longiceps carry the stripped seeds back out of the nest. Usually the discarded seeds are deposited on the surrounding mound where they soon become buried in sand – the ants are continually adding newly-excavated soil to the nest mound. Sometimes the ants stash the discarded seeds further from the mound, beneath leaves or sticks (Berg 1975).
This provides a good place to grow. The mound soil into which the seeds are discarded is nutrient enriched, enhancing seedling growth (Anderson 1988).
Many of our local plants are adapted for ant-dispersal.
Myrmecochorous plants are a distinctive feature of heath and dry sclerophyll forests in south-eastern Australia (Berg 1975, Rice & Westoby 1981). Most are shrubs growing on infertile, well-drained soils. Sounds very much like our place!
Two types of evidence have been used to identify myrmecochorous plants: direct observation of ants carrying the seeds back to their nests; and inference based on the presence of a seed appendage in one species that is “exactly similar” to that of a known, myrmecochorous relative (Berg 1975). On this basis, local myrmecochorous species include many of our common and widespread shrubs, forbs and creepers: most of our wattles (e.g. Acacia terminalis, A. longifolia, A. mearnsii, A. obtusifolia); Amperea xiphoclada; Beyeria lasiocarpa; Bossiaea cordifolia; Dodonea triquetra; Goodenia ovata; Hardenbergia violacea; Hovea heterophylla; Kennedia rubicunda; Lepidosperma sieberi; Monotoca scoparia; Pultenaea daphnoides; Schelhammera undulata; Tetratheca pilosa; Thysanotus tuberosus; Xanthosia pilosa. And there are almost certainly others.
Ants, fire and recovery
These little ants have also played an important role in the post-fire recovery of the forest.
Many of the seedlings that appeared in the weeks and months after the January 2020 bushfire are myrmecochorous and so probably sprouted from ant-buried seeds.
Wattle (Acacia sp.) seedling within 4 weeks of the fire (26/1/2020)
Kennedia rubicunda seedlings. Within a year, this creeper had become a prominent feature of the post-fire landscape (28/1/2020)
For a detailed look at post-fire regrowth, see Paul’s posts: The forest: five seasons on (April 2021), and Forest vegetation - another year on (May 2022)
The ant colonies themselves survived the fire. When I look back over our early, post-fire photos I am reminded of the high levels of ant activity –including funnel-shaped mounds and several photos that are clearly Aphaenogaster longiceps, uncharacteristically active during the day.
Of course, many individual ants would have died. But studies have shown that Aphaenogaster longiceps has a high thermal tolerance (Hemmings & Andrew 2017) and that during bushfire they survive in their deep tunnel systems (Shakesby et al. 2007). Indeed, their mounding activity is actually reported to increase in the aftermath of fire (Shakesby et al. 2007).
Their burrowing activities may have helped feed the soil. Burrowing ants such as Aphaenogaster longiceps play an important role in soil mixing (Richards 2009). They bring sediments from around 30cm below ground to the soil surface, and in turn bury sediments and leaf litter that has accumulated on the forest floor (Richards 2009).
Such ‘bioturbation’ was particularly evident in the immediate aftermath of the fire, as the grey and white ash was progressively overlayed by the unburnt soil of the nest mounds.
Ash and char is high in most plant nutrient elements, including Ca, K, and Mg (Yusiharni & Gilkes 2012), and so may have had a fertilising effect. However, the importance of this ash-bed in terms of plant nutrition is not easily predicted. Soil pH, mineral solubility, and species-specific requirements are among the many factors involved.
All I can say with any certainty is that the activity of the ants did change the colour of the forest floor in the early days post-fire!
They also helped save the forest from erosion. Very heavy rains in the immediate aftermath of bushfire can lead to loss of topsoil and scouring. We certainly had plenty of opportunity for such erosion in early 2020. Within ten weeks of the fire we had received nearly 300mm of rain, much of it falling as intense downpours.
The burrows and mounds of Aphaenogaster longiceps – and no doubt other ant species – helped reduce the velocity of overground water flow, and channel water underground (Richards 2009, Richards et al. 2011, Shakesby et al. 2007).
The uneven, porous surface created by ant mounds impedes the flow of water over the ground surface. Scorched canopy leaves that fell in the weeks following the fire also play a role, forming ‘litter-dams’.
(28/1/2020)
It should be stressed, however, that the ants were just one factor saving the forest from widespread soil loss. In the forests of Australia’s south-east, the rapid recovery of vegetation probably plays a critical role – both through direct protection of the soil and increasingly rates of transpiration (Shakesby et al. 2007). Soil digging by returning birds and the scorched leaf litter fall are also recognised as important.
Third, that spectacular breeding swarm
So back to that breeding event of October 2021.
It was a calm afternoon at the end of a particularly hot day when the air suddenly filled with large, shiny ants. We’re very familiar with fluttering swarms of winged termites … this was different. The insects’ flight was slow, heavy and laboured. Tracking individuals was easy. They were rarely aloft for long, they flew neither high nor far.
I followed the pair in the image below, coupled in flight. They came to ground in an open, sandy area and remained joined for a few seconds. In the short time it took me to focus my camera on them, they separated.
Pair of Aphaenogaster longiceps alates, immediately after landing and uncoupling. The large female (left) is at least 10mm long, while the slender male (right) was not much larger than the workers, at 5-6mm. (18/10/2021)
Emergence
While I was chasing mated pairs, Paul headed off to see if he could find the source. On a hunch, he checked along one of our sandy trails where we had previously spotted extensive ant diggings …. and he was right! Processions of large, winged ants were emerging from several adjacent holes and low mounds, apparently harried by much smaller workers.
Over the following hour, we watched dozens of winged queens emerge from this and adjacent openings. Curiously, we did not see a single male among them. Either the males emerged earlier, or the males I was seeing in flight had arisen from colonies elsewhere in the forest. Although this was the only emergence site we found, the huge aerial swarms indicated a synchronised swarming from many different nests.
The queens didn’t take to the air immediately. Instead they hauled themselves up nearby plants before becoming airborne. A large body, previously untested wings and flight muscles … makes sense to start with a jump off.
The aerial phase
Aerially, the swarm appeared to congregate in an open area of the forest around our house, just a few metres above ground. It is possible that the house clearing (or even the house itself) created a focal point, drawing alates from surrounding colonies. Such landscape-based aggregation has been described in other species of ants (Wilson 1957).
Many of the flying females I tracked in flight had a tiny male attached and so I assume that mating of Aphaenogaster longiceps is an airborne affair. Copulation may include a final phase on the ground … pairs briefly remained joined after landing, but only for a few seconds.
Back to earth
Soon after landing, females shed their wings. This is a deliberate act involving considerable dexterity!
A newly-mated queen lands and starts to remove her wings. Here one hind wing falls, having just been broken off.
The wings don’t simply fall off or break when the ant starts to burrow (as they do in termites). Stretching her legs and twisting her body, the mated queen breaks off each wing in turn.
The series below shows the same ant removing her final wing. She seems intent on the operation, ignoring both me and my camera as I crouch alongside.
Within a couple of minutes she is rid of the encumbrance and free to seek a sandy place to found a new colony.
Another queen, same dance, this time in realtime video (90 seconds).
A short life for many
Successful ant queens can live for years (Antwiki - colony foundation). Soon after mating and shedding their wings, they find a secluded spot underground, break down their now-redundant flight muscles and start egg production.
Of course, very few females become founding queens. The vast majority simply become food for others – highlighting yet one more contribution these ants make to the forest ecosystem.
Swarming conditions
The swarming didn’t last long. We first noticed it around 5pm and it was all over within two hours. And it must have involved multiple colonies – there were thousands of insects in flight. Such synchronicity implies a common cue spanning a large area. Weather is the most likely candidate.
Rainfall and temperature are often implicated as triggers in the synchronised swarming of ants (Markin et al. 1971, Obin & Vander Meer 1994, Rhoades & Davis 1967). Fortunately, we have detailed data from our home weather station.
Of particular note:
Between 14th and 16th October, we recorded 25mm rain, most of it falling in just 3 hours.
On 16th October, atmospheric pressure reached a low point of 995 hPa after falling steadily for six days.
The day of the swarm – 18th October – was hotter than usual. It reached a maximum of 25 degrees C and was over 20 degrees from 10am to 4:30pm. During the late afternoon swarming it was still above 18 degrees … and a few spots of rain started to fall.
Atmospheric pressure dropped steadily during the 6 hours leading up to the swarming, reaching a low point at 4pm.
Rain fell in earnest just 8 hours later – over 11mm by 8am.
Weather conditions during October 2021, as recorded here in the forest. Total rainfall for the month was 93mm across 11 rain days. [Note that the rainfall shown in blue is cumulative for each 24 hour period].
So I suspect that conditions in the week leading up to the event ‘primed’ the colony, and the final trigger – the one that truly synchronised swarming across the forest – was the drop in atmospheric pressure on the 18th. The barometer reached a low point after 3pm before starting to rise again within an hour. And we first noticed the aerial swarm shortly before 5pm. Just an idea …
Weather conditions on the day of the swarming, including temperature, precipitation and atmospheric pressure.
The backstage story
What else was going on below ground in the lead up to the swarming? When did the alates emerge from their pupal cases? What roles did the workers play? Where were the colony’s males? And how did the females know when it was time to shed their wings?
My search for descriptions of the breeding behaviour of Aphaenogaster longiceps has proven fruitless so far. Despite the notoriety of the species, its breeding biology may be as yet undescribed. Just another reminder that with the study of nature there is always something more to learn.
However, I did have some luck. The Red Fire Ant (Solenopsis invicta) is a native of Brazil but now a major pest in the United States and an ongoing threat for Australia. For this reason it is perhaps the best-studied ant species, particularly in terms of alate behaviour and nuptial flight regulation (Burns et al. 2007, Fletcher & Blum 1983, Obin & Vander Meer 1994, Vargo & Laurel 1994). The reason I feel particularly fortunate is that Solenopsis and Aphaenogaster are related (both belong to the subfamily Myrmicinae) and both form short-lived aerial swarms of small males and larger females. It is therefore likely that the two species share other aspects of breeding regulation and behaviour.
So extrapolating from these fire ant studies, I have developed a hypothetical narrative for the funnel ant swarm. While only a theoretical scenario, it is a reasoned one.
‘Birth of an Aphaenogaster longiceps queen’
This is the tale of a young queen, just one among many.
Alongside her winged sisters and brothers, both older and younger, she rests safe in her underground home.
Workers come and go, keeping her groomed and fed.
Her mother, the colony’s reigning queen, emits volatile chemicals which permeate the tunnels. The young female samples them with her waving antennae, unaware that she is being doped. Her maturation is slowed, the development of her eggs put on hold. And she ignores the cumbersome wings draping her back.
While the young queen and her winged siblings rest, life in the colony continues. The old queen lays fertilised eggs while her numerous worker daughters busily forage, tend the larvae, and maintain the nest.
It is now mid October and the ground temperature is rising. Rain falls, the soil is moist, humidity in the nest builds. Atmospheric pressure falls.
The young queen responds. She sends the workers a chemical ‘call to action’. They all now have a different job to do!
Workers clear the nest openings and check outside for threats. Then, in response to an external, forest-wide cue, they usher the winged males to the surface.
With her brothers gone, it is now our young queen’s turn. She recruits her own escort, a retinue of workers that fussily guide her out of the nest and toward nearby vegetation. But only when conditions are right. If the workers deem it too windy or sense predators nearby, they pull her back to the safety of the burrow.
Eventually she clambers up a low bush, while her escort remains on standby. They will step in to help if she has a problem.
Safely atop her chosen plant, she is ready to go. Warm sun, a small puff of air – and she takes flight!
The light breeze helps her gain height but she’s strong enough to choose her own direction. And there’s the clue … a scent that lures her toward the waiting swarm of males.
A few metres above the ground, in an open section of forest, males from many colonies circle and look out for arriving females.
Entering this swarm, she is joined by a male. They mate in flight. She soon spies what looks a good place to nest and spirals down to land.
Her mate leaves shortly afterward, his job now done. She carries all the sperm she will need to fertilise her eggs for years to come.
Having mated, she knows the time has come to get rid of her wings. One by one, she breaks them off at the base.
Now landbound, she walks off alone.
Her redundant wing muscles quickly start to break down, recycled for the production of eggs. Soon she will found a dynasty of her own.
Finally, a nest invasion
In the weeks following the swarming event, as I pondered all we had seen, I regularly dropped by the site of the original colony. All was quiet. In fact, the burrows used by the emerging queens were closed. Nearby were fresh, funnel-shaped mounds clearly belonging to Aphaenogaster longiceps – perhaps even the same colony – but I saw no daytime activity.
That all changed on 6th November!
Ants were milling about the nest entrances in large numbers, clearly agitated. I soon discovered the cause. Not a swarming event. This time – rove beetles! Large, winged adults and flightless but mobile grubs, running around the ant mounds, moving in and out of the burrows … and clearly drawing the ants’ attention.
I was puzzled. Were the beetles predators or prey?
I did a bit more reading, this time about rove beetles.
Rove beetles (family Staphylinidae) are one of the largest families of beetles – and include more ant-associating species than any other beetle family (Parker 2016). They seem pre-adapted to take advantage of the social fabric of ant societies. Most rove beetles have elongated, highly flexible bodies, are adapted to life on or in the soil, and (like all beetles) their membranous flight wings are protected by a pair of tough elytra.
Initially I was quite excited, thinking we had stumbled across a case of symbiosis or social parasitism. Perhaps the beetles laid their own eggs within the ants’ nest! But after a bit more reading, and looking back over the photos, I think a more mundane explanation may suffice.
I have tentatively identified the beetles as genus Thyreocephalus. Published descriptions of this genus and its near relatives describe them as “highly motile, specialised predators” (Lawrence & Britton 1991, p624). Indeed, we often see these beetles hunting among gatherings of dung-feeders – insects and other arthropods. So I guess the rove beetles were simply attempting to raid the funnel ant nests and hunting insect scavengers on the surrounding middens.
The series of images below shows an adult beetle entering a burrow only to reemerge within seconds, pursued by ants. The scoreboard for this encounter? RAIDER 0: DEFENDERS 1.
Yet something still troubles me. The ants didn’t seem to put up enough of a fight, particularly against the grubs. In fact, at times it was hard to tell if they were trying to drive the beetles away, pull them down into the burrow – or indeed groom the grubs! So maybe there was more going on than I can guess. Some mystery remains.
Whatever drove the engagement, it didn’t last long. I visited the site regularly over the following weeks, and the ants had resumed their secretive ways. Rove beetles were still active across the forest, but were no longer concentrated around the ants’ nests.
REFERENCES
Anderson, A.N. 1988. Soil of the nest-mound of the seed-dispersing ant, Aphaenogaster longiceps, enhances seedling growth. Australian Journal of Ecology, 12: 469-471
Berg. 1975. Myrmecochorous plants in Australia and their dispersal by ants. Australian Journal of Botany, 23: 475-508
Burns, S.N., Vander Meer, R.K. & Teal, P.E.A. 2007. Mating flight activity as dealation factors for red imported fire ant (Hymenoptera: Formicidae) female alates. Annals of the Entomological Society of America, 100(2): 257-264
Fletcher, D.J.C. & Blum, M.S. 1983. The inhibitory pheromone of queen fire ants: effects of disinhibition on dealation and oviposition by virgin queens. Journal of Comparative Physiology, 153: 467-475
Hemmings, Z. & Andrew, N.R. 2017. Effects of microclimate and species identity on body temperature and thermal tolerance of ants (Hymenoptera: Formicidae). Austral Entomology, 56: 104-114
Hughes, L. & Westoby, 1992. Effect of diaspore characteristics on removal of seeds adapted for dispersal by ants. Ecology, 73(4): 1300-1312
Hughes, L., Westoby, M. & Jurado, E. 1994. Convergence of elaisomes and insect prey: evidence from ant foraging behaviour and fatty acid composition. Functional Ecology, 8: 358-365
Lawrence, J.F. & Britton, E.B. 1991. Coleoptera (Beetles), Chapter 35 in The Insects of Australia. Division of Entomology, CSIRO. Melbourne University Press.
Markin, G.P., Dillier, J.H., Hill, S.O., Blum, M.S. & Hermann, H.R. 1971. Nuptial flight and flight ranges of the imported fire ant, Solenopsis saevissima richteri (Hymenoptera: Formicidae). Journal of the Georgia Entomological Society, 6(3): 145 (abstract)
Obin. M.S. and Vander Meer, R.K. 1994. Alate semiochemicals release worker behavior during fire ant nuptial flights. Journal of Entomological Science, 29(1); 143-151
Parker, J. 2016. Myrmecophily in beetles (Coleoptera): evolutionary patterns and biological mechanisms. Myrmecological News, 22: 65-108
Rhoades, W.C. & Davis, D.R. 1967. Effects of meteorological factors on the biology and control of the imported fire ant. Journal of Economic Entomology, 60(2): 554 (abstract)
Rice, B. & Westoby, M. 1981. Myrmecochory in sclerophyll vegetation of the West Head, New South Wales. Australian Journal of Ecology, 6: 291-298
Richards, P.J. 2009. Aphaenogaster ants as bioturbators: impacts on soil and slope processes. Earth-Science Reviews, 2009: 92-206
Richards, P.J., Humphreys, G.S., Tomkins, K.M., Shakesby, R.A. & Doerr, S.H. 2011. Bioturbation on wildfire-affected southeast Australian hillslopes: spatial and temporal variation. Catena, 87: 20-30
Shakesby, R.A., Wallbrink, P.J., Doerr, S.H., English, P.M., Chafer, C.J., Humphreys, G.S., Blake, W.H. & Tomkins, K.M. 2007. Distinctiveness of wildfire effects on soil erosion in south-east Australian eucalypt forests assessed in a global context. Forest Ecology and Management, 238: 347-364
Shattuck, S.O. 2008. Australian ants of the genus Aphaenogaster (Hymenoptera: Formicidae). Zootaxa, 1677: 25-45
Vargo, E.L. & Laurel, M. 1994. Studies on the mode of action of a queen primer pheromone of the fire ant Solenopsis invicta. Journal of Insect Physiology, 40(7); 601-610
Wilson, E. O. 1957. The organization of a nuptial flight of the ant Pheidole sitarches Wheeler. Psyche, 64(2): 46-50
Yusiharni, E. & Gilkes, R. 2012. Minerals in the ash of Australian native plants. Geoderma, 189-190: 369-380