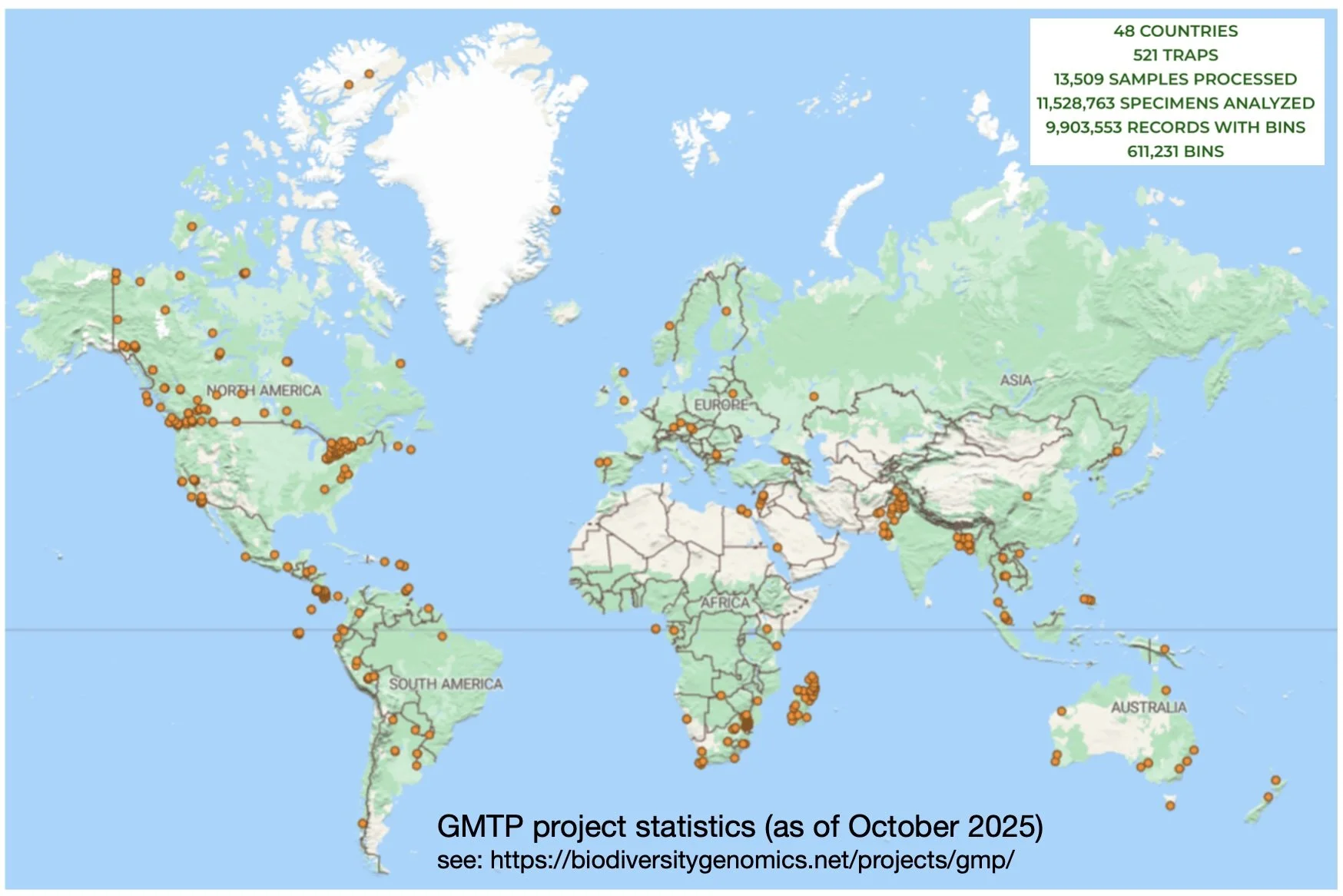
GMP coverage, as of October 2025
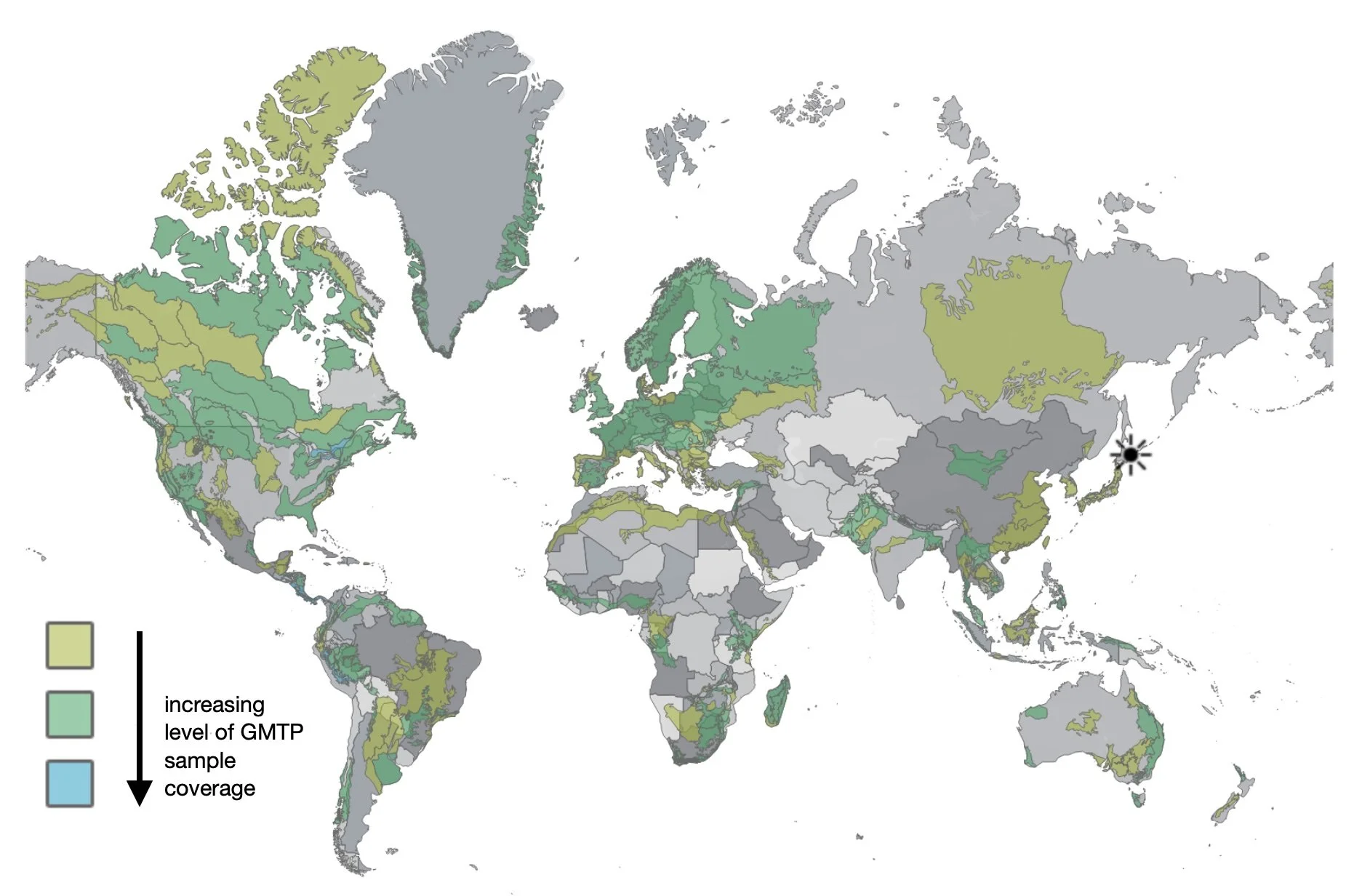
Ecoregions currently sampled (with at least 10,000 records and 1,000 BINS) as part of the GMP.
https://public.tableau.com/app/profile/evgeny.zakharov/viz/EcoRegionCoveragePublic/EcoRegionCoverage

Trap 1, one of two Malaise traps we erected on 3rd January 2026. Both will be in place for the next 12 months. The elevation at this site is the highest across our home forest, about 26m above sea level.

Trap 2, on a track that runs through a low-lying part of the forest (~16m above sea level). The barrier surrounding it is to discourage wombats and wallabies from barging on through. After all, the traps do straddle the forest tracks regularly used by the local wildlife … and by us.

Within a couple of hours of initial set-up, it was clear Trap 1 was working! Insects collect in the upper chamber and are preserved in the ethanol filled collection bottle.

Here’s a glimpse of the first week’s harvest from Trap 1. Even we were amazed by the extraordinary diversity! Granted, conditions were particularly ‘good’ for insects that week, with above average temperatures. A heatwave, in fact.
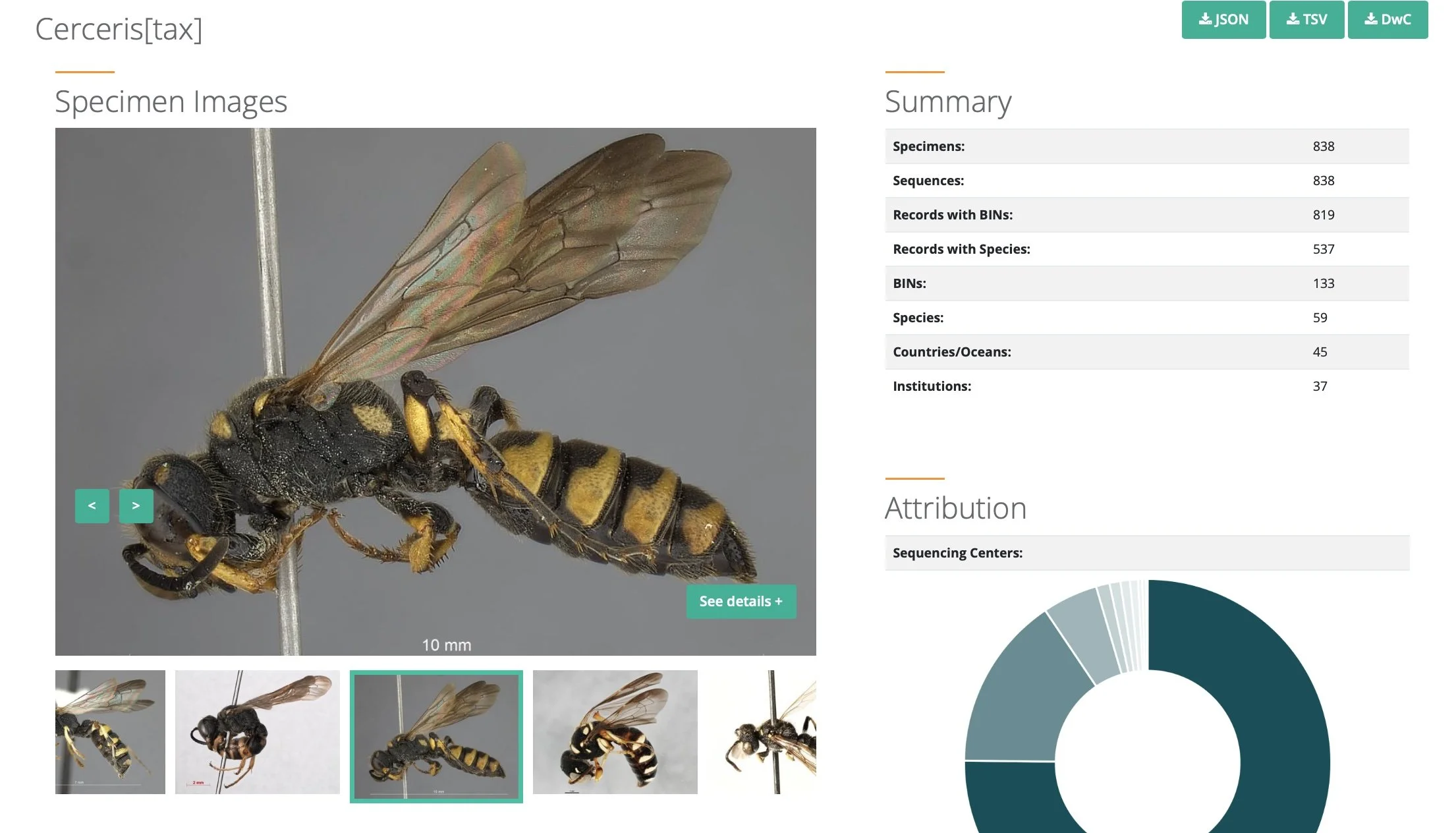
The BOLD library currently holds barcode data for more than 800 specimens of Cerceris, spanning 45 across Europe, Africa, North America, Asia … and a few from Australia. These represent 133 species, based on their DNA barcodes.
https://portal.boldsystems.org/result?query=Cerceris[tax]
![Currently, filtering the search for Cerceris in Australia yields 19 specimens, representing 8 species (as determined by their DNA barcodes … BINS in this summary table). https://portal.boldsystems.org/result?query=Cerceris[tax],Australia[geo]](https://images.squarespace-cdn.com/content/v1/58ec80a8d2b857fe42e4f603/1769731670493-H3PI4JH761F0HJNC9BQD/Cerceris+in+Australia+BOLD.jpg)
Currently, filtering the search for Cerceris in Australia yields 19 specimens, representing 8 species (as determined by their DNA barcodes … BINS in this summary table).
https://portal.boldsystems.org/result?query=Cerceris[tax],Australia[geo]
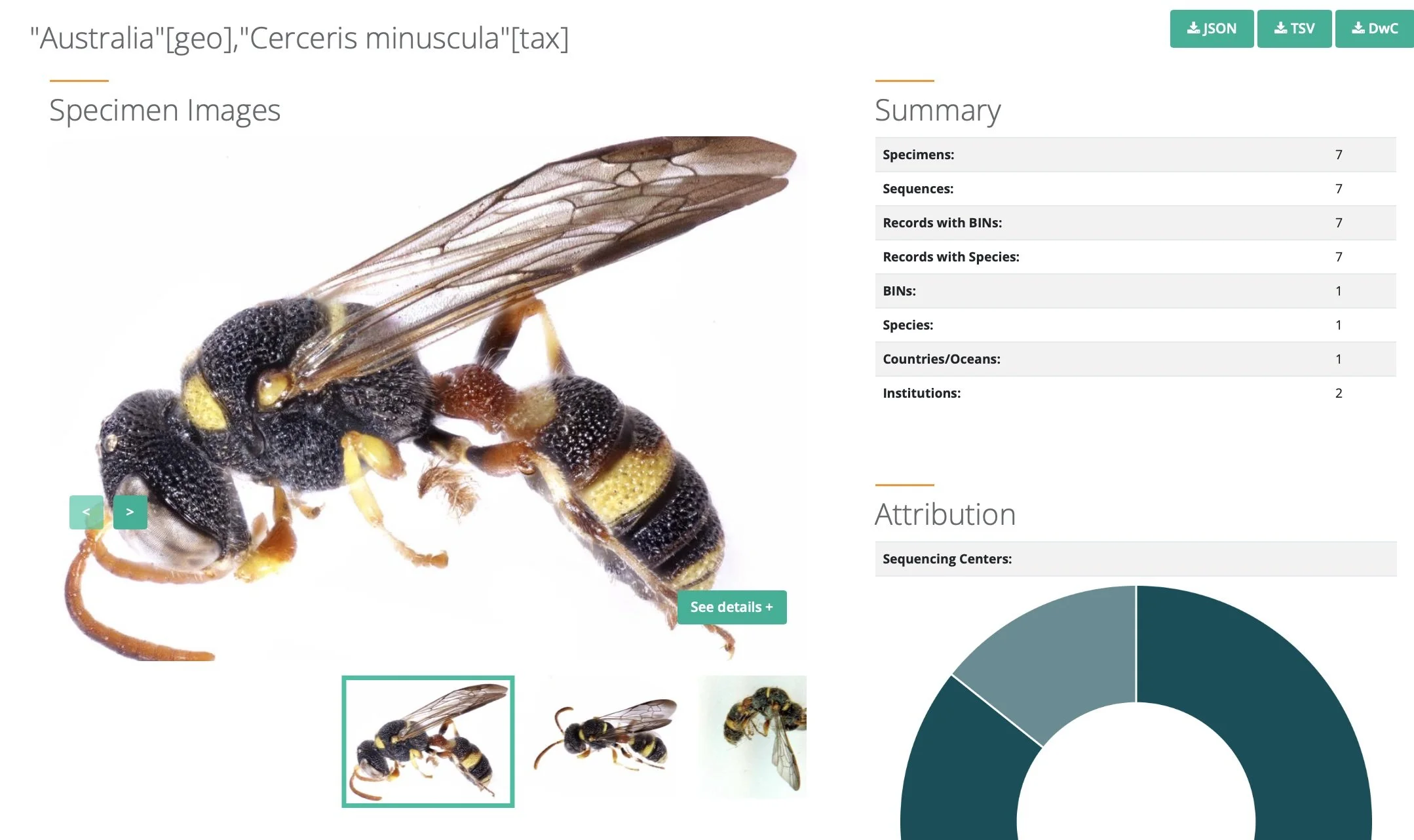
For one species of Cerceris, Cerceris minuscula, there are currently 7 records. The specifics of where, when and how each specimen was collected are all recorded on BOLD, along with other relevant details. This information is freely available to the public (with the exception of any data under embargo while awaiting publication by the researchers involved).
https://portal.boldsystems.org/result?query=%22Cerceris%20minuscula%22[tax]
![Another example of search results by genus, this one a page Paul has made good use of in his ongoing study of Australian sawflies. https://portal.boldsystems.org/result?query=Perga[tax]](https://images.squarespace-cdn.com/content/v1/58ec80a8d2b857fe42e4f603/1769731681281-JRVLXH6NG6RKB9J6MZ9H/Perga+BOLD.jpg)
Another example of search results by genus, this one a page Paul has made good use of in his ongoing study of Australian sawflies.
https://portal.boldsystems.org/result?query=Perga[tax]

The GMP employs the same Malaise trap design, worldwide. For example:
top left: Mt. Kitanglad Range, PHILIPPINES
middle left: SINT EUSTATIUS (Dutch Caribbean island)
middle right: Ziarat, PAKISTAN
bottom left: Beirut, LEBANON
bottom right: Isabela Island, GALAPAGOS / ECUADOR
[images from GMP website]

The aim is to set the trap across an insect flight path. The GMP sampling protocol includes the following explanation:
Site Selection - Deploy the trap at a site which is subject to minimal disturbance and ideally in a habitat’s climax vegetation (i.e. placement in a national park or other protected area is preferred). When possible, position the trap at a right angle to an insect flight line, in areas with low undergrowth; forest edges or clearings and elevated sites are recommended.
![Samples are collected weekly and stored cold (ideally in a freezer). Batches are then shipped to the Centre for Biodiversity Genomics for imaging and sequencing. [image from GMP website ]](https://images.squarespace-cdn.com/content/v1/58ec80a8d2b857fe42e4f603/1769736454642-3FQ4H8JBIZR6U8LF00BR/sample+bags.jpg)
Samples are collected weekly and stored cold (ideally in a freezer). Batches are then shipped to the Centre for Biodiversity Genomics for imaging and sequencing.
[image from GMP website]

The black barrier net of the trap is set across the insect flight path. Many flying insects move towards light when they encounter an obstacle. Hence the white roof of the tent.
The Malaise trap was initially developed by René Malaise, back in 1934. The version in use across the GMP is a later model, known as Malaise Trap II, Townes Style.
photo: Trap 1, 30th Jan. 2026
![At CBG, the samples are analysed in detail. This includes a system for imaging individual specimens before sequencing. [extract Steinke et al. 2024, Figure 1, p. 122]](https://images.squarespace-cdn.com/content/v1/58ec80a8d2b857fe42e4f603/1769736463211-1LMLUJWU96NN68Q0501I/Steinke+Fig+1+extract.jpg)
At CBG, the samples are analysed in detail. This includes a system for imaging individual specimens before sequencing.
[extract Steinke et al. 2024, Figure 1, p. 122]

Once inside the tent, all upward movement leads to the collection opening at the apex.
photo: Trap 1, 30th Jan. 2026

Once an insect ‘escapes’ the tent through the apex opening, it is trapped in the collecting chamber. Most soon drop into the ethanol filled bottle below.
photo: Trap 1, 30th Jan. 2026
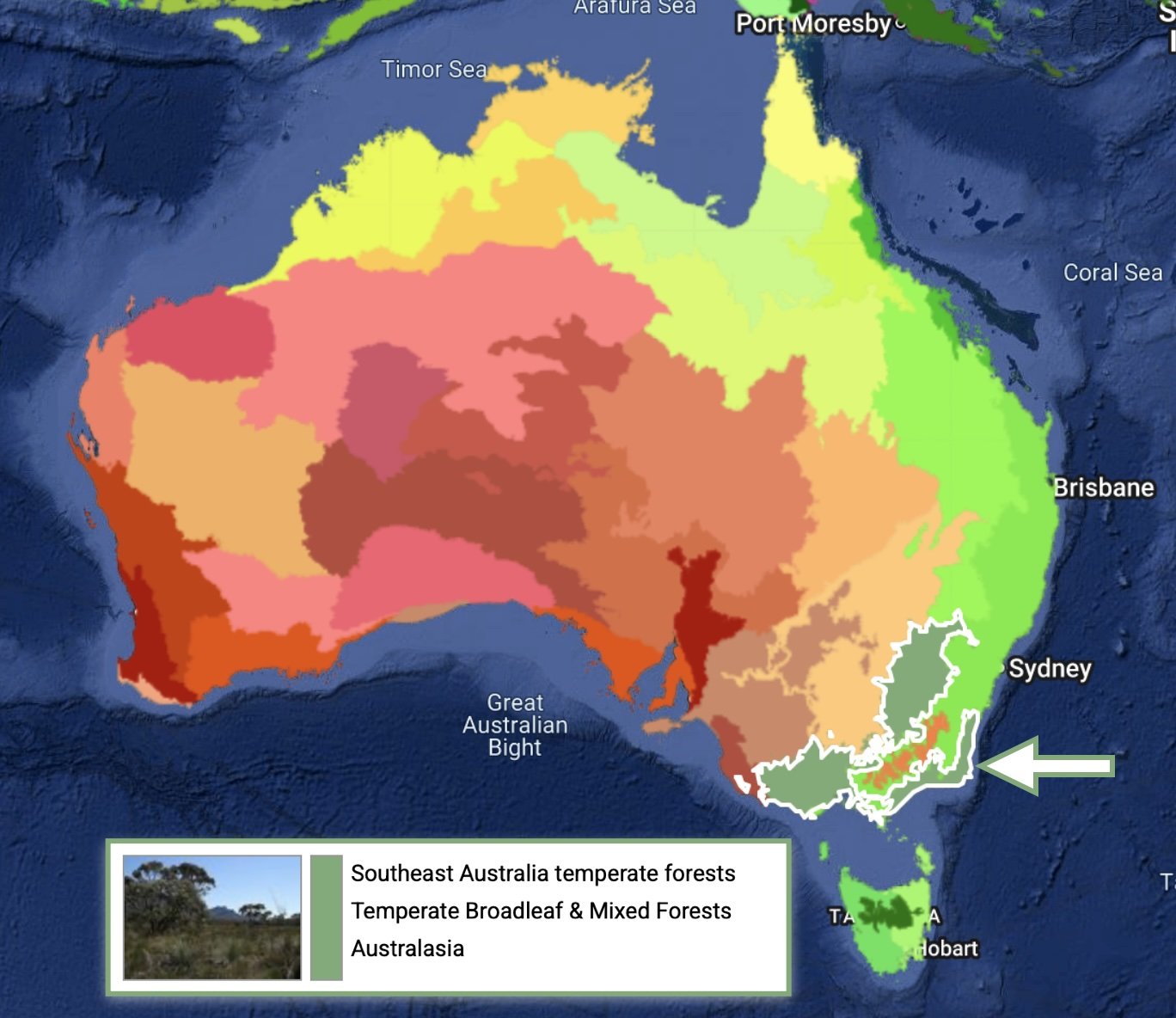
We are located in a GMP ecoregion (as per Dinerstein et al. 2017) that includes the far south coast of NSW, the east coast of Victoria, a large part of south western Victoria, and south western slopes of NSW.
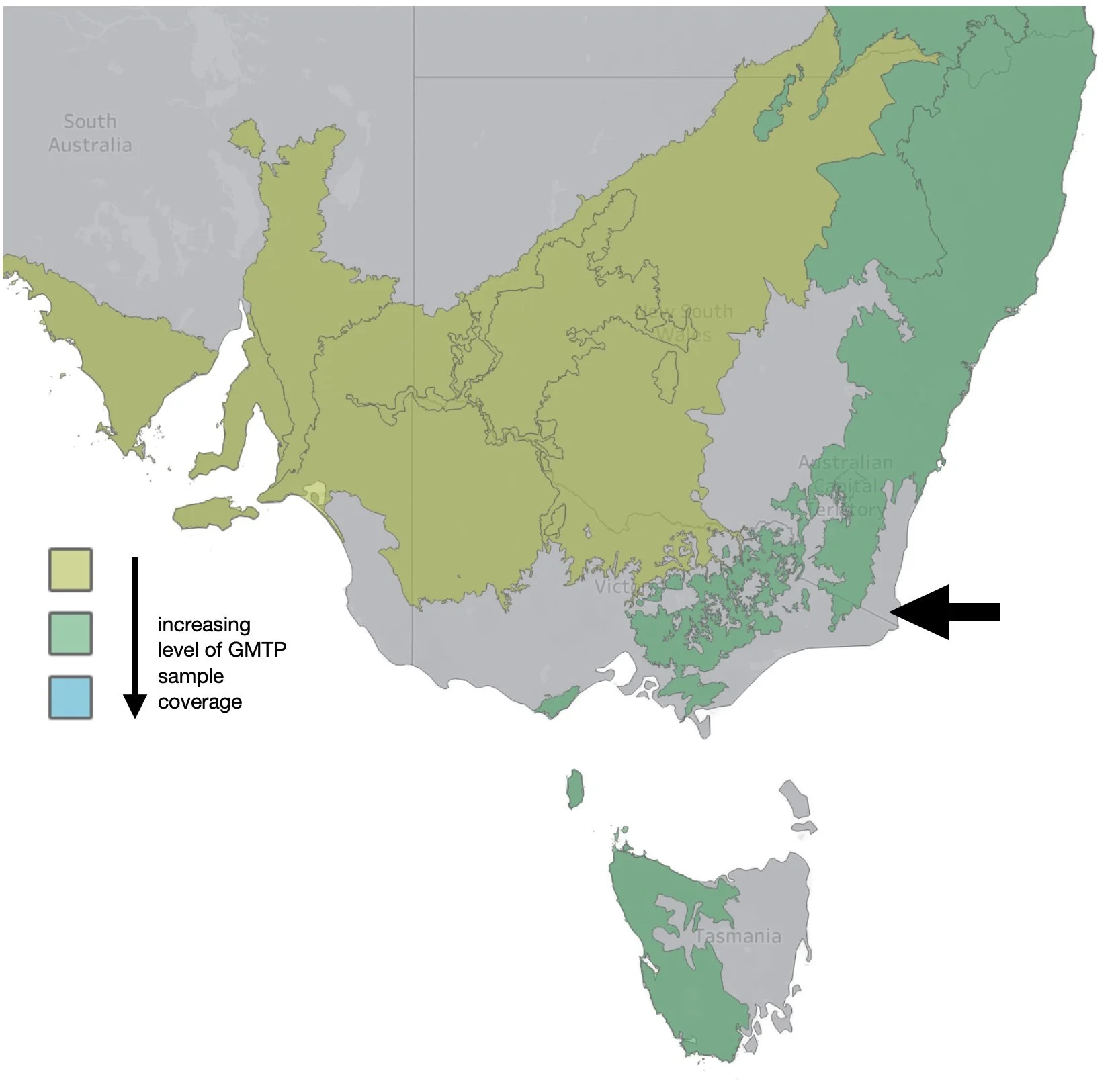
Our samples will be the first for our ecoregion!

Austrogorytes spryi
There are currently no reference examples on BOLD for this endemic Australian genus.
Collected here by hand, 30th December 2025. Currently stored dry, pinned. (#2512E)

Sphodrotes punctuosa
Another Australian endemic genus not well represented in the BOLD reference collection is Sphodrotes. There are currently 5 specimens, and all a single species – S. nemoralis.
Having examined our local species in detail, I’m confident it is a different species – S. punctuosa.
Collected here by hand, 24th December 2025 (#2512C). Currently stored in ethanol, but soon to be pinned.

Now this is one of several rather special specimens – genus Megalyra.
The family Megalyridae is currently represented by just 22 specimens on BOLD, and the genus Megalyra just 7 – from anywhere in the world. With just one from Australia!
Collected here by hand, 19th August 2024. Currently stored dry. (#2408B)

Proctotrupidae I think, based on the venation and ovipositor. BOLD currently has just 2 specimens of a single Australian species, recognised at the family level. This may be the same species … but it may be different. The barcode will be the test.
Either way, it’s a new family for our home list! No wonder really, given its small size.
(Temporarily isolated from the Trap 1, Week 2 collection, with special permission from Paul Hebert.)

This looks like Pseudoturneria, a crabronid wasp I’ve studied nearby but until now had not found here in the forest. Once I set and pin it, I’ll be better placed to confirm the ID.
Pseudoturneria is yet another Australian genus not currently represented in the BOLD database. We’ll soon change that!
From Trap 1, Week 1.

Now who is this intriguing crabronid?!? At first glance I thought another Pseudoturneria … but perhaps not. The legs are a different colour, so it’s almost certainly a different species. Definitely worth a detailed study before it goes off to BOLD for sequencing.
From Trap 1, Week 3.

The missing female!
Arrolla lawrencei was described from a single male, the female unknown at that time (1990). We have sighted pairs of this species here in the past (details here), but not collected them. It seems we have now! A description of the female is worthy of a short publication … and barcoding, of course!
From Trap 1, Week 3

Flies and wasps dominate each of our weekly collections. Many are large and showy, and there is a huge variety. Others are extremely small – but they all play a role in the forest ecosystem. And all will be barcoded.
With special permission, we view each sample collection before bagging and storage. Any specimens removed for further study are carefully labelled, and we take great care in handling. It is critical to the validity of the GMP data that every insect, no matter how small, is recorded and processed as part of the appropriate sample.

It’s not ALL flies, wasps, ants and bees. Many other orders are represented in the mix. In this image alone there are various beetles (Coleoptera), bugs (Hemiptera), moths (Lepidoptera), a cockroach (Blattodea) and grasshoppers (Orthoptera).

The raspy cricket in the centre of this image was a surprising find. Not only do we see this species only rarely … but it is flightless. It must have climbed the trap mesh and ultimately fallen into the collection bottle. And it wasn’t alone! This single sample contained an adult female (centre, arrow), an adult male, and at least five nymphs (eg arrow top left).
Note too the many tiny insects, some circled in red. Probably flies and wasps … we’ll know soon enough!

Another postcard for biodiversity. By my reckoning there are six insect orders in shot here, and apart from a few beetles, no two are alike.
With a pair of small spiders for extra variety.
I didn’t arrange them this way. It’s just a random closeup of a part of the (much!) larger weekly sample.







![Currently, filtering the search for Cerceris in Australia yields 19 specimens, representing 8 species (as determined by their DNA barcodes … BINS in this summary table). https://portal.boldsystems.org/result?query=Cerceris[tax],Australia[geo]](https://images.squarespace-cdn.com/content/v1/58ec80a8d2b857fe42e4f603/1769731670493-H3PI4JH761F0HJNC9BQD/Cerceris+in+Australia+BOLD.jpg)

![Another example of search results by genus, this one a page Paul has made good use of in his ongoing study of Australian sawflies. https://portal.boldsystems.org/result?query=Perga[tax]](https://images.squarespace-cdn.com/content/v1/58ec80a8d2b857fe42e4f603/1769731681281-JRVLXH6NG6RKB9J6MZ9H/Perga+BOLD.jpg)


![Samples are collected weekly and stored cold (ideally in a freezer). Batches are then shipped to the Centre for Biodiversity Genomics for imaging and sequencing. [image from GMP website ]](https://images.squarespace-cdn.com/content/v1/58ec80a8d2b857fe42e4f603/1769736454642-3FQ4H8JBIZR6U8LF00BR/sample+bags.jpg)

![At CBG, the samples are analysed in detail. This includes a system for imaging individual specimens before sequencing. [extract Steinke et al. 2024, Figure 1, p. 122]](https://images.squarespace-cdn.com/content/v1/58ec80a8d2b857fe42e4f603/1769736463211-1LMLUJWU96NN68Q0501I/Steinke+Fig+1+extract.jpg)















