Grass Tree sea

We have long marvelled at the sea of grass trees that dominates a patch of our home forest. We have wondered why they grow so densely in just that one area. We have celebrated each flower spike, as rare as they are extraordinary. Since The Fire, their leafy growth has been astonishing – a source of encouragement and wonder. We keenly anticipate a flowering bonanza this Spring.
It is widely accepted that Xanthorrhoea respond well after fire, often flowering in profusion. But I realise how little I really know about our local species. Will our plants respond in this way? And what does the future hold for this population, given the fundamental changes to the forest in the wake of The Fire?
Identifying our species
There are around 30 species of Xanthorrhoea, all native to Australia. They are iconic plants, well known for their tussocks of grass-like leaves atop blackened trunks (hence the common name, ‘grass trees’) and their tall, persistent flower spikes.
I’m reasonably confident that our home species is Xanthorrhoea concava.
Unlike the archetypal grass tree, our local species has no visible trunk. The leafy tussocks arise from the soil or, at most, from atop a ground-level crown of dead leaf bases. The plant does have a stem, but it’s below ground. This is surprisingly common – of the 13 Xanthorrhoea species in NSW, only 5 produce an obvious trunk.
An unusually large crown of dead leaf bases suggests this is quite an old plant.
There are just four candidate species here in the south-east corner of the country and three of these have subterranean stems: X. resinosa, X. concava, and X. minor. Unlike X. resinosa (ref #3), our plants have multiple crowns due to branching of the trunk. The dimensions of the flower spike are a good match for X. concava. We’ll take a close look at the flowers this Spring, to be sure.
Impressive survival and regrowth
The grass trees are the stand-out survivors of last Summer’s bushfire.
Despite careful searching, I’ve found very few dead plants. It’s possible that some burnt away without trace, but I doubt that we lost many. Although a few plants are damaged and may yet lose a branch, most live on. The photos below show four of the worst affected plants.
Such a high survival rate is not unexpected. It accords with results for X. resinosa near Sydney (ref #1). Their fire-resilience is the result of a well-protected apical meristem, at the centre of the tussock. The surrounding leaf bases shield it from heat damage. The meristem is where new leaves and flower spikes originate.
The flush of new growth we now see is a combination of two classes of leaves.
First are those that were burned by the fire but have been able to keep growing. These are the leaves with the brown tips. The growth of these leaves, through the continual production of new tissue at the base, restored the plant’s photosynthetic capacity within weeks of the fire. The grass trees had a head start on all other plants.
Second are the new leaves produced from the apical meristem. Stored starch reserves in the plant stem fuel the rapid production and growth of these fresh leaves at the centre of each tussock. The majority of leaves we see now are of this class.
Fire has a fertilising effect (ref #2). Minerals such as potassium, calcium, magnesium and boron are ash-derived and highly soluble. Luckily we had about 20mm rain within days of the fire, and nearly 200mm by the end of February. The Xanthorrhoea took advantage of the boon. Their roots will have absorbed these nutrients, fuelling the first flush of growth and also building a store of minerals in the stem for use in future leaf production – and in flowering!
The older leaves are progressively dying as they are replaced by the new growth. This is normal. Nutrients from the dead leaves have been returned to the plant while the dry, brown leaves contribute to the leaf litter surrounding the crowns. Minerals such as sodium and potassium are quickly leached into the soil (ref #2). Others, such as magnesium, calcium and boron tend to be retained – until burnt in the next fire!
The oldest leaves are shed as new leaves are produced.
This burst of regrowth belies the true nature of grass trees. Xanthorrhoea are slow-growing and long-lived plants. Leaf turnover can be rapid and huge flowering spikes appear to grow overnight, but the actual plants take years to develop a sizeable stem and may live for centuries. For example:
“… X. resinosa is likely to require 40 – 60 years to mature and from 120 – 240 years following germination before significant reproductive output occurs” (Tozer & Keith, 2012, pB265).
On this basis, our largest grass trees may be much older than our largest eucalypts. I find that astonishing.
Impressive numbers
The sheer number of plants in the patch is quite staggering. There are literally thousands. We counted the tussocks in each of two 10 metre by 10 metre plots. There were more than 200 tussocks per 100 square metres! And the patch covers around 5,000 square metres of our home forest (but more about that later).
Obtaining an accurate count of the number of plants is tricky. An individual plant may have multiple crowns. Some tussocks are tightly grouped and clearly belong to one plant, but many are single and well-spaced. Based on an average of three tussocks per plant (probably an over estimate) that still corresponds to thousands of individual plants across the patch.
Evidence of a branched underground stem … two tussocks close together.
The flowering has begun
There was much excitement this week when we discovered the first nascent flower spikes. Just eight spikes so far, on just four plants. But it’s encouraging! Peak flowering is November-December, so there’s plenty of time.
Flower spikes is an inaccurate term, botanically. I should describe the flowering in terms of a ‘spike’ and a ‘scape’. The spike is the end where the flowers will form, the scape is the stem below it.
At this stage, the spikes are very early in their growth and development.
Immature flower spike atop a thick, photosynthetic scape (on a rainy morning)
All being well, the spikes will continue to lengthen, and so will the scapes. At maturity, the spike and scape are roughly equal in length, collectively over 2 metres tall!
In full flower, December 2017. This plant is growing near the road. The area was slashed by Council just months before – I suspect the disturbance triggered the growth spurt and flowering.
Flowering is expensive. Studies have shown that a single spike can cost the equivalent of 223 leaves (ref #2). We can only hope that our plants have sufficient stored resources to put on a display this Spring. Time will tell.
For our grass trees, fire was overdue
Our grass tree patch may have been unburnt for 40 years. We know that the last major fire to impact the forest was in 1980. Research on X. resinosa indicates that such long periods between fires may be detrimental to a population, over time (ref #1). Fire plays a direct role by stimulating flowering, and flowering is the only means by which new plants are generated. There are also indirect effects. In the absence of fire, surrounding shrubs may overgrow and shade the grass trees, limiting their growth and ultimate fire-response capacity (ref #1).
I’m not too concerned about our patch. The flush of growth we see now suggests the plants are well-fed. If they can produce such huge numbers of leaves, so quickly, indications are they will have the resources necessary to successfully flower and fruit.
In addition, the plants were not overgrown. They are in open forest, with a scattering of middle storey plants. At least there was, before the fire. This contrasts with the situation for X. resinosa which grows in heath, where dense shrub thickets form in the absence of frequent fire.
The grass tree patch, several years ago.
Nevertheless, a good flowering season this year will be reassuring. The scattered seeds will help replace lost plants and perhaps even allow the patch to spread into adjacent areas.
A restricted patch
Curiously, the grass trees are concentrated in this one particular area. There are scattered plants nearby, but not many and not in such density.
The patch extends across the highest part of our property. It continues onto the neighbouring bush block and also a short distance into adjacent State Forest. The entire area is perhaps 15,000 square metres (at a guess), with a third of that being within our home boundaries.
The soil is sandy and well-drained. Most of the patch is in rather open forest. The main trees are eucalypts (mostly Eucalyptus sieberi), with large banksias (Banksia serrata) dominating the eastern corner.
Why do they grow in this one patch? I still do not have an answer. I suspect soil type. An exposed rocky cliff nearby suggests that the underlying rock may be rhyolite, an igneous rock formed from larva. I think. Perhaps this distinguishes the soil from the surrounding sedimentary sand. Maybe.
Drainage may also be involved. The site is higher than the surrounds. But this fails to account for the absence of grass trees on forested ridges nearby.
Maybe it’s also historical. Xanthorrhoea grow very slowly and they live a long time. Perhaps past land use and fire patterns have left their mark.
It is a special patch. Whatever the reason for the existence of the patch, we have reason to believe that it’s rather special. A local botanist (and good friend) commented that she’s rarely seen such a concentration of this species. As I move through the surrounding forests I’ve been on the lookout – after The Fire, Xanthorrhoea are particularly easy to spot! Yet I’ve seen very few.
Another moth survivor
Paul recently wrote a blog about emerging ghost moths, protected during the fire as underground caterpillars. The story below is a similar tale of survival.
We have long known that our Xanthorrhoea play host to a rather large species of moth, Ptilomacra senex. The adults emerge in Winter and males are attracted to light.
In recent weeks these males have been common visitors to our house windows.
They are quite beautiful insects. Striking markings, iridescent scales … and the most extraordinary antennae!
Ptilomacra senex … 24/5/20
Ptilomacra senex belongs to the family Cossidae. Cossids are commonly called wood moths as their larvae feed by burrowing into woody plants. The caterpillars of Ptilomacra senex burrow within the stems of Xanthorrhoea, specifically. The adults we are seeing now would have been oblivious caterpillars as fire razed the forest in January.
The next generation is underway. We found newly-laid eggs on two Xanthorrhoea plants this week. We are confident of the identity as they are an exact match for published images of this species. And they are on Xanthorrhoea.
The shiny, dark eggs are laid in tight, spiralling clusters around leaf blades.
Upon hatching, the tiny caterpillars will disperse and find their way to new host plants. They’ll burrow into the stem and remain there until they emerge as adults. The feeding of these caterpillars no doubt takes a toll on the plants, but presumably it is a sustainable relationship. We have lots of moths and yet lots of flourishing grass trees!
Research
I’ve been trawling the literature on ‘Xanthorrhoea and fire’ to see what I could learn. A detailed study of X. resinosa in the Royal National Park near Sydney is particularly relevant (ref #1). Xanthorrhoea resinosa and X. concava are alike – they were originally considered a single species – so they may respond similarly to fire. A second paper describing the physiological response of Xanthorrhoea to fire is also informative, although the authors studied an arboreal species of Western Australia (ref #2). I’ve yet to find very much ecological information specific to X. concava.
References
Tozer, M.G. & Keith, D.A. 2012. Population dynamics of Xanthorrhoea resinosa Pers. over two decades: implications for fire management. Proceedings of the Linnean Society of New South Wales 134, B249-B266.
Lamont, B.B., Wittkuhn, R. & Korczynskyj, D. 2004. Turner Review No. 8. Ecology and ecophysiology of grasstrees. Australian Journal of Botany, 52, 561-582
Flora of Australia Volume 46, Iridaceae to Dioscoreaceae, Australian Government Publishing Service, Canberra (1986)
[unless stated, all photos above were taken in August 2020]
Update: Identity check Late October
When Kerri wrote this post in mid August, she was reasonably confident about which Xanthorrhoea species we have in our forest.
She decided that X. concava is the most likely candidate, based on the following features:
the stem is entirely subterranean, with no sign of a trunk above ground
plants often have multiple stems due to branching of the trunk below the ground, as shown in the example below. (The woody parts protruding above the surface in these photos are the bases of burnt leaves - not trunks).
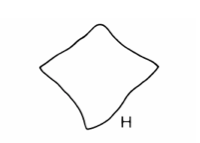
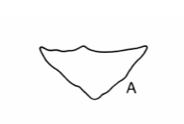
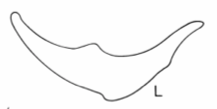
The shape of the leaves provides another line of evidence. The diagrams below, taken from ref #3, show leaf shapes in cross-section for the three species with subterranean trunks in our region. (We used the key in this reference to arrive at the species identity).
This photo shows a leaf from one of our Xanthorrhoea plants cut in cross section.
It appears to be halfway between X. concava and X. minor. It doesn’t show the pronounced concavity of X. concava but it is more elongated than X. minor.
However the authors of ref #3 state that leaf shape is a rather variable character, changing somewhat from plant to plant and along the length of the leaf.
The inflorescence provides more reliable characters for species identification. So we were waiting impatiently for flowers to develop on our grass tree spikes. During the last fortnight, that has begun to take place, as shown in the photos below.
The flowering spikes, which have presumably attained their final length, are shorter than, or at the most, the same height as the scape. This is consistent with a species identity of X. concava.
The inflorescence provides other useful characters - in particular, the detailed structure of the following parts:
packing bracts, the highly modified leaves that fill the spaces between the flowers.
sepals and petals of the flowers themselves.
First, a word about petals and sepals.
Xanthorrhoea flowers are highly modified.
I found it quite difficult to even recognise the sepals and petals at first. These lie in two whorls - an outer whorl of 3 sepals and an inner whorl of 3 petals - first forming a tiny cap over the developing floral parts. The arrowhead in this photo points to a single sepal.
As the stamens and style develop, the sepals and petals are pushed aside - as shown in the lower flower.
They remain as tiny plates (arrowed), flanking the male and female flower parts.
You can see the arrangement of 6 stamens, the single central style and the sepals/petals in fully developed flowers in the photo “closeup of emerging flowers” above. (You’ll need to zoom in to see the sepals!).
Xanthorrhoea species differ in the detailed structure of the packing bracts, sepals and petals. To visualise theses features, I cut out a couple of flowers from a spike and imaged them under the microscope.
The structure of these flower parts supports a species identity of X. concava.
the packing bracts (C.) are ‘shortly acute’. This means that their tips are a bit pointier than rounded but are not sharply pointed.
the outer side of the packing bracts is covered in pale hairs and the tip with brown hairs. This gives the spike a velvety, light brown appearance. The X. resinosa spike, in comparison, is dark brown - a product of the dark colour of the hairs on its packing bracts.
The outer face of the sepal has many pale hairs (D.). The tips are shortly acute.
The petals (E.) are ‘reflexed’, meaning that the tips are bent over slightly. They are glabrous (smooth), except for a tuft of short hairs at the tip.